A lack of immune system genes causes loss in high frequency hearing but does not disrupt cochlear synapse maturation in mice
- PMID: 24804771
- PMCID: PMC4012943
- DOI: 10.1371/journal.pone.0094549
A lack of immune system genes causes loss in high frequency hearing but does not disrupt cochlear synapse maturation in mice
Abstract
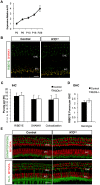
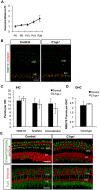
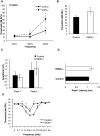
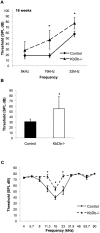
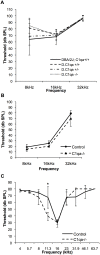
Early cochlear development is marked by an exuberant outgrowth of neurites that innervate multiple targets. The establishment of mature cochlear neural circuits is, however, dependent on the pruning of inappropriate axons and synaptic connections. Such refinement also occurs in the central nervous system (CNS), and recently, genes ordinarily associated with immune and inflammatory processes have been shown to play roles in synaptic pruning in the brain. These molecules include the major histocompatibility complex class I (MHCI) genes, H2-K(b) and H2-D(b), and the complement cascade gene, C1qa. Since the mechanisms involved in synaptic refinement in the cochlea are not well understood, we investigated whether these immune system genes may be involved in this process and whether they are required for normal hearing function. Here we report that these genes are not necessary for normal synapse formation and refinement in the mouse cochlea. We further demonstrate that C1qa expression is not necessary for normal hearing in mice but the lack of expression of H2-K(b) and H2-D(b) causes hearing impairment. These data underscore the importance of the highly polymorphic family of MHCI genes in hearing in mice and also suggest that factors and mechanisms regulating synaptic refinement in the cochlea may be distinct from those in the CNS.
Conflict of interest statement
Figures

Similar articles
-
Thyroid hormone controls the timing of cochlear ribbon synapse maturation.Biochem Biophys Res Commun. 2024 Apr 16;704:149704. doi: 10.1016/j.bbrc.2024.149704. Epub 2024 Feb 21. Biochem Biophys Res Commun. 2024. PMID: 38430700
-
Timed conditional null of connexin26 in mice reveals temporary requirements of connexin26 in key cochlear developmental events before the onset of hearing.Neurobiol Dis. 2015 Jan;73:418-27. doi: 10.1016/j.nbd.2014.09.005. Epub 2014 Sep 22. Neurobiol Dis. 2015. PMID: 25251605
-
Autophagy-Mediated Synaptic Refinement and Auditory Neural Pruning Contribute to Ribbon Synaptic Maturity in the Developing Cochlea.Front Mol Neurosci. 2022 Mar 4;15:850035. doi: 10.3389/fnmol.2022.850035. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35310883 Free PMC article.
-
Major histocompatibility complex class I proteins in brain development and plasticity.Trends Neurosci. 2012 Nov;35(11):660-70. doi: 10.1016/j.tins.2012.08.001. Epub 2012 Aug 30. Trends Neurosci. 2012. PMID: 22939644 Free PMC article. Review.
-
Purinergic Signaling and Cochlear Injury-Targeting the Immune System?Int J Mol Sci. 2019 Jun 18;20(12):2979. doi: 10.3390/ijms20122979. Int J Mol Sci. 2019. PMID: 31216722 Free PMC article. Review.
Cited by
-
Interactions between Macrophages and the Sensory Cells of the Inner Ear.Cold Spring Harb Perspect Med. 2019 Jun 3;9(6):a033555. doi: 10.1101/cshperspect.a033555. Cold Spring Harb Perspect Med. 2019. PMID: 30181352 Free PMC article. Review.
-
Cochlear Immune Response in Presbyacusis: a Focus on Dysregulation of Macrophage Activity.J Assoc Res Otolaryngol. 2022 Feb;23(1):1-16. doi: 10.1007/s10162-021-00819-x. Epub 2021 Oct 12. J Assoc Res Otolaryngol. 2022. PMID: 34642854 Free PMC article. Review.
-
Macrophage-Mediated Glial Cell Elimination in the Postnatal Mouse Cochlea.Front Mol Neurosci. 2017 Dec 11;10:407. doi: 10.3389/fnmol.2017.00407. eCollection 2017. Front Mol Neurosci. 2017. PMID: 29375297 Free PMC article.
-
Facial Nerve Recovery in KbDb and C1q Knockout Mice: A Role for Histocompatibility Complex 1.Plast Reconstr Surg Glob Open. 2016 Dec 23;4(12):e1186. doi: 10.1097/GOX.0000000000001186. eCollection 2016 Dec. Plast Reconstr Surg Glob Open. 2016. PMID: 28293529 Free PMC article.
-
C1q as a target molecule to treat human disease: What do mouse studies teach us?Front Immunol. 2022 Aug 3;13:958273. doi: 10.3389/fimmu.2022.958273. eCollection 2022. Front Immunol. 2022. PMID: 35990646 Free PMC article.
References
-
- Lenoir M, Shnerson A, Pujol R (1980) Cochlear receptor development in the rat with emphasis on synaptogenesis. Anat Embryol 160: 253–262. - PubMed
-
- Bruce LL, Kingsley J, Nichols DH, Fritzsch B (1997) The development of vestibulocochlear efferents and cochlear afferents in mice. Int J Dev Neurosci 15: 671–692. - PubMed
-
- Simmons DD, Moulding HD, Zee D (1996) Olivocochlear innervation of inner and outer hair cells during postnatal maturation: an immunocytochemical study. Brain Res Dev Brain Res 95: 213–226. - PubMed
-
- Cole KS, Robertson D (1992) Early efferent innervation of the developing rat cochlea studied with a carbocyanine dye. Brain Res 575: 223–230. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

