Striatal neurodevelopment is dysregulated in purine metabolism deficiency and impacts DARPP-32, BDNF/TrkB expression and signaling: new insights on the molecular and cellular basis of Lesch-Nyhan Syndrome
- PMID: 24804781
- PMCID: PMC4013014
- DOI: 10.1371/journal.pone.0096575
Striatal neurodevelopment is dysregulated in purine metabolism deficiency and impacts DARPP-32, BDNF/TrkB expression and signaling: new insights on the molecular and cellular basis of Lesch-Nyhan Syndrome
Abstract
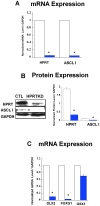
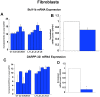
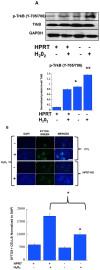
Lesch-Nyhan Syndrome (LNS) is a neurodevelopmental disorder caused by mutations in the gene encoding the purine metabolic enzyme hypoxanthine-guanine phosphoribosyltransferase (HPRT). This syndrome is characterized by an array of severe neurological impairments that in part originate from striatal dysfunctions. However, the molecular and cellular mechanisms underlying these dysfunctions remain largely unidentified. In this report, we demonstrate that HPRT-deficiency causes dysregulated expression of key genes essential for striatal patterning, most notably the striatally-enriched transcription factor B-cell leukemia 11b (Bcl11b). The data also reveal that the down-regulated expression of Bcl11b in HPRT-deficient immortalized mouse striatal (STHdh) neural stem cells is accompanied by aberrant expression of some of its transcriptional partners and other striatally-enriched genes, including the gene encoding dopamine- and cAMP-regulated phosphoprotein 32, (DARPP-32). Furthermore, we demonstrate that components of the BDNF/TrkB signaling, a known activator of DARPP-32 striatal expression and effector of Bcl11b transcriptional activation are markedly increased in HPRT-deficient cells and in the striatum of HPRT knockout mouse. Consequently, the HPRT-deficient cells display superior protection against reactive oxygen species (ROS)-mediated cell death upon exposure to hydrogen peroxide. These findings suggest that the purine metabolic defect caused by HPRT-deficiency, while it may provide neuroprotection to striatal neurons, affects key genes and signaling pathways that may underlie the neuropathogenesis of LNS.
Conflict of interest statement
Figures





Similar articles
-
Deficiency of the purine metabolic gene HPRT dysregulates microRNA-17 family cluster and guanine-based cellular functions: a role for EPAC in Lesch-Nyhan syndrome.Hum Mol Genet. 2013 Nov 15;22(22):4502-15. doi: 10.1093/hmg/ddt298. Epub 2013 Jun 25. Hum Mol Genet. 2013. PMID: 23804752 Free PMC article.
-
A Golgi study of neuronal architecture in a genetic mouse model for Lesch-Nyhan disease.Neurobiol Dis. 2005 Nov;20(2):479-90. doi: 10.1016/j.nbd.2005.04.005. Neurobiol Dis. 2005. PMID: 15908225
-
MicroRNA-mediated dysregulation of neural developmental genes in HPRT deficiency: clues for Lesch-Nyhan disease?Hum Mol Genet. 2012 Feb 1;21(3):609-22. doi: 10.1093/hmg/ddr495. Epub 2011 Oct 31. Hum Mol Genet. 2012. PMID: 22042773 Free PMC article.
-
Hypothesized deficiency of guanine-based purines may contribute to abnormalities of neurodevelopment, neuromodulation, and neurotransmission in Lesch-Nyhan syndrome.Clin Neuropharmacol. 2005 Jan-Feb;28(1):28-37. doi: 10.1097/01.wnf.0000152043.36198.25. Clin Neuropharmacol. 2005. PMID: 15711436 Review.
-
[Complete and partial deficiency of HPRT].Nihon Rinsho. 1996 Dec;54(12):3315-20. Nihon Rinsho. 1996. PMID: 8976112 Review. Japanese.
Cited by
-
ASCL1-regulated DARPP-32 and t-DARPP stimulate small cell lung cancer growth and neuroendocrine tumour cell proliferation.Br J Cancer. 2020 Sep;123(5):819-832. doi: 10.1038/s41416-020-0923-6. Epub 2020 Jun 5. Br J Cancer. 2020. PMID: 32499571 Free PMC article.
-
Purine Metabolism and Dystonia: Perspectives of a Long-Promised Relationship.Ann Neurol. 2025 May;97(5):809-825. doi: 10.1002/ana.27227. Epub 2025 Mar 3. Ann Neurol. 2025. PMID: 40026236 Free PMC article. Review.
-
Hypoxanthine deregulates genes involved in early neuronal development. Implications in Lesch-Nyhan disease pathogenesis.J Inherit Metab Dis. 2015 Nov;38(6):1109-18. doi: 10.1007/s10545-015-9854-4. Epub 2015 May 5. J Inherit Metab Dis. 2015. PMID: 25940910
-
The interrelationship of metabolic syndrome and neurodegenerative diseases with focus on brain-derived neurotrophic factor (BDNF): Kill two birds with one stone.Metab Brain Dis. 2017 Jun;32(3):651-665. doi: 10.1007/s11011-017-9997-0. Epub 2017 Mar 30. Metab Brain Dis. 2017. PMID: 28361262 Review.
-
Emerging Role of Purine Metabolizing Enzymes in Brain Function and Tumors.Int J Mol Sci. 2018 Nov 14;19(11):3598. doi: 10.3390/ijms19113598. Int J Mol Sci. 2018. PMID: 30441833 Free PMC article. Review.
References
-
- Jinnah HA, Friedmann T (2000) Lesch-Nyhan Disease and its variants. In: Scriver C, Beaudet, AL, Sly WS, Valle, D., editor. The Metabolic and Molecular bases of inherited disease. 8 ed ed: McGraw-Hill. 2537–2570.
-
- Lesch M, Nyhan WL (1964) A Familial Disorder of Uric Acid Metabolism and Central Nervous System Function. Am J Med 36: 561–570. - PubMed
-
- Lloyd KG, Hornykiewicz O, Davidson L, Shannak K, Farley I, et al. (1981) Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N Engl J Med 305: 1106–1111. - PubMed
-
- Ernst M, Zametkin AJ, Matochik JA, Pascualvaca D, Jons PH, et al. (1996) Presynaptic dopaminergic deficits in Lesch-Nyhan disease. N Engl J Med 334: 1568–1572. - PubMed
-
- Jinnah HA, Langlais PJ, Friedmann T (1992) Functional analysis of brain dopamine systems in a genetic mouse model of Lesch-Nyhan syndrome. JPharmacolExpTher 263: 596–607. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

