Vaccines against invasive Salmonella disease: current status and future directions
- PMID: 24804797
- PMCID: PMC4185946
- DOI: 10.4161/hv.29054
Vaccines against invasive Salmonella disease: current status and future directions
Abstract
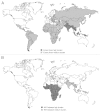
Though primarily enteric pathogens, Salmonellae are responsible for a considerable yet under-appreciated global burden of invasive disease. In South and South-East Asia, this manifests as enteric fever caused by serovars Typhi and Paratyphi A. In sub-Saharan Africa, a similar disease burden results from invasive nontyphoidal Salmonellae, principally serovars Typhimurium and Enteritidis. The existing Ty21a live-attenuated and Vi capsular polysaccharide vaccines target S. Typhi and are not effective in young children where the burden of invasive Salmonella disease is highest. After years of lack of investment in new Salmonella vaccines, recent times have seen increased interest in the area led by emerging-market manufacturers, global health vaccine institutes and academic partners. New glycoconjugate vaccines against S. Typhi are becoming available with similar vaccines against other invasive serovars in development. With other new vaccines under investigation, including live-attenuated, protein-based and GMMA vaccines, now is an exciting time for the Salmonella vaccine field.
Keywords: GMMA; Salmonella; enteric; global health; glycoconjugate; nontyphoidal; typhoid; vaccines.
Figures

References
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–22. doi: 10.1016/S0140-6736(13)60844-2. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
