Induction of mixed chimerism using combinatory cell-based immune modulation with mesenchymal stem cells and regulatory T cells for solid-organ transplant tolerance
- PMID: 24804993
- PMCID: PMC4172387
- DOI: 10.1089/scd.2013.0617
Induction of mixed chimerism using combinatory cell-based immune modulation with mesenchymal stem cells and regulatory T cells for solid-organ transplant tolerance
Abstract
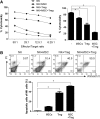
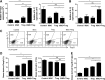
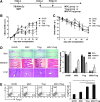
Establishment of mixed chimerism is an ideal approach to induce donor-specific tolerance while expanding its potential in various clinical settings. Despite the developments in partial conditioning regimens, improvements are still needed in reducing toxicity and bone marrow transplantation-related complications. Recently, cell-based therapies, including mesenchymal stem cells (MSCs), have been incorporated in establishing noncytoreductive mixed chimerism protocols; however, its efficacy is only partial and shows reversed immunosuppressive properties. This study demonstrates a novel approach to induce mixed chimerism and tolerance through combinatory cell-based immune modulation (CCIM) of MSCs and regulatory T cells (Tregs). We hypothesize that the interaction between these cells may lead to greater inhibition of host immune responses. Compared with single cell therapy, CCIM induced a higher engraftment rate and robust donor-specific tolerance to skin allografts across full major histocompatibility complex barriers. These regulatory effects were associated with inhibition of natural killer cell cytotoxic activity, CD4(+)IL-17(+) cells, memory B cells, plasma cells, and immunoglobulin production levels along with increased frequencies of CD4(+)Foxp3(+) cells, IL-10-producing mature B cells, and myeloid-derived suppressor cells. Furthermore, CCIM was able to regulate mortality in a graft-versus-host disease model through reciprocal regulation of Treg/Th17. Taken together, we suggest CCIM as a clinically applicable strategy for facilitating the induction of mixed chimerism and permanent tolerance.
Figures




Similar articles
-
Mesenchymal stem cells enhance the induction of mixed chimerism and tolerance to rat hind-limb allografts after bone marrow transplantation.J Surg Res. 2010 May 15;160(2):315-24. doi: 10.1016/j.jss.2008.09.027. Epub 2008 Nov 4. J Surg Res. 2010. PMID: 19524257
-
Induction of allogeneic mixed chimerism by immature dendritic cells and bone marrow transplantation leads to prolonged tolerance to major histocompatibility complex disparate allografts.Immunology. 2009 Aug;127(4):500-11. doi: 10.1111/j.1365-2567.2009.03057.x. Immunology. 2009. PMID: 19604303 Free PMC article.
-
Bone marrow transplantation combined with mesenchymal stem cells induces immune tolerance without cytotoxic conditioning.J Surg Res. 2011 Nov;171(1):e123-31. doi: 10.1016/j.jss.2011.06.020. Epub 2011 Jul 13. J Surg Res. 2011. PMID: 21920556
-
Facilitating cells as a venue to establish mixed chimerism and tolerance.Pediatr Transplant. 2003 Oct;7(5):348-57. doi: 10.1034/j.1399-3046.2003.00100.x. Pediatr Transplant. 2003. PMID: 14738294 Review.
-
A Large-Scale Bank of Organ Donor Bone Marrow and Matched Mesenchymal Stem Cells for Promoting Immunomodulation and Transplant Tolerance.Front Immunol. 2021 Feb 26;12:622604. doi: 10.3389/fimmu.2021.622604. eCollection 2021. Front Immunol. 2021. PMID: 33732244 Free PMC article. Review.
Cited by
-
Myeloid-derived suppressor cells therapy enhance immunoregulatory properties in acute graft versus host disease with combination of regulatory T cells.J Transl Med. 2020 Dec 14;18(1):483. doi: 10.1186/s12967-020-02657-6. J Transl Med. 2020. PMID: 33317573 Free PMC article.
-
Autologous Mesenchymal Stem Cells for Treatment of Chronic Active Antibody-Mediated Kidney Graft Rejection: Report of the Phase I/II Clinical Trial Case Series.Transpl Int. 2022 Nov 22;35:10772. doi: 10.3389/ti.2022.10772. eCollection 2022. Transpl Int. 2022. PMID: 36484064 Free PMC article. Clinical Trial.
-
Combining Adoptive Treg Transfer with Bone Marrow Transplantation for Transplantation Tolerance.Curr Transplant Rep. 2017;4(4):253-261. doi: 10.1007/s40472-017-0164-7. Epub 2017 Nov 4. Curr Transplant Rep. 2017. PMID: 29201599 Free PMC article. Review.
-
New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation.Int J Stem Cells. 2015 May;8(1):54-68. doi: 10.15283/ijsc.2015.8.1.54. Int J Stem Cells. 2015. PMID: 26019755 Free PMC article. Review.
-
Enhanced immunoregulation of mesenchymal stem cells by IL-10-producing type 1 regulatory T cells in collagen-induced arthritis.Sci Rep. 2016 Jun 1;6:26851. doi: 10.1038/srep26851. Sci Rep. 2016. PMID: 27246365 Free PMC article.
References
-
- Copelan EA. (2006). Hematopoietic stem-cell transplantation. N Engl J Med 354:1813–1826 - PubMed
-
- Welniak LA, Blazar BR. and Murphy WJ. (2007). Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol 25:139–170 - PubMed
-
- Pilat N. and Wekerle T. (2010). Mechanistic and therapeutic role of regulatory T cells in tolerance through mixed chimerism. Curr Opin Organ Transplant 15:725–730 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

