Quantitative flux analysis reveals folate-dependent NADPH production
- PMID: 24805240
- PMCID: PMC4104482
- DOI: 10.1038/nature13236
Quantitative flux analysis reveals folate-dependent NADPH production
Erratum in
- Nature. 2014 Sep 25;513(7519):574
Abstract
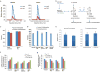
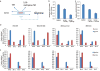
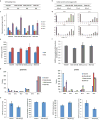
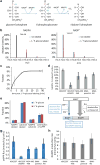
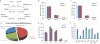
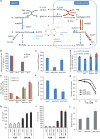
ATP is the dominant energy source in animals for mechanical and electrical work (for example, muscle contraction or neuronal firing). For chemical work, there is an equally important role for NADPH, which powers redox defence and reductive biosynthesis. The most direct route to produce NADPH from glucose is the oxidative pentose phosphate pathway, with malic enzyme sometimes also important. Although the relative contribution of glycolysis and oxidative phosphorylation to ATP production has been extensively analysed, similar analysis of NADPH metabolism has been lacking. Here we demonstrate the ability to directly track, by liquid chromatography-mass spectrometry, the passage of deuterium from labelled substrates into NADPH, and combine this approach with carbon labelling and mathematical modelling to measure NADPH fluxes. In proliferating cells, the largest contributor to cytosolic NADPH is the oxidative pentose phosphate pathway. Surprisingly, a nearly comparable contribution comes from serine-driven one-carbon metabolism, in which oxidation of methylene tetrahydrofolate to 10-formyl-tetrahydrofolate is coupled to reduction of NADP(+) to NADPH. Moreover, tracing of mitochondrial one-carbon metabolism revealed complete oxidation of 10-formyl-tetrahydrofolate to make NADPH. As folate metabolism has not previously been considered an NADPH producer, confirmation of its functional significance was undertaken through knockdown of methylenetetrahydrofolate dehydrogenase (MTHFD) genes. Depletion of either the cytosolic or mitochondrial MTHFD isozyme resulted in decreased cellular NADPH/NADP(+) and reduced/oxidized glutathione ratios (GSH/GSSG) and increased cell sensitivity to oxidative stress. Thus, although the importance of folate metabolism for proliferating cells has been long recognized and attributed to its function of producing one-carbon units for nucleic acid synthesis, another crucial function of this pathway is generating reducing power.
Figures







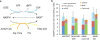
References
-
- Voet DV, Voet JG. Biochemistry. 3rd. John Wiley & Sons; 2004.
-
- Lee WN, et al. Mass isotopomer study of the nonoxidative pathways of the pentose cycle with [1,2-13C2] glucose. Am J Physiol. 1998;274:E843–851. - PubMed
-
- Brekke EM, Walls AB, Schousboe A, Waagepetersen HS, Sonnewald U. Quantitative importance of the pentose phosphate pathway determined by incorporation of 13C from [2-13C]- and [3-13C] glucose into TCA cycle intermediates and neurotransmitter amino acids in functionally intact neurons. J Cereb Blood Flow Metab. 2012;32:1788–1799. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

