Transmural intestinal wall permeability in severe ischemia after enteral protease inhibition
- PMID: 24805256
- PMCID: PMC4013012
- DOI: 10.1371/journal.pone.0096655
Transmural intestinal wall permeability in severe ischemia after enteral protease inhibition
Abstract
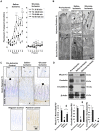
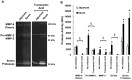
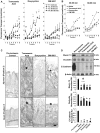
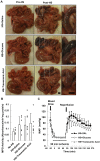
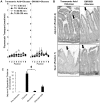
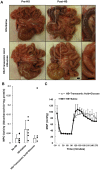
In intestinal ischemia, inflammatory mediators in the small intestine's lumen such as food byproducts, bacteria, and digestive enzymes leak into the peritoneal space, lymph, and circulation, but the mechanisms by which the intestinal wall permeability initially increases are not well defined. We hypothesize that wall protease activity (independent of luminal proteases) and apoptosis contribute to the increased transmural permeability of the intestine's wall in an acutely ischemic small intestine. To model intestinal ischemia, the proximal jejunum to the distal ileum in the rat was excised, the lumen was rapidly flushed with saline to remove luminal contents, sectioned into equal length segments, and filled with a tracer (fluorescein) in saline, glucose, or protease inhibitors. The transmural fluorescein transport was determined over 2 hours. Villi structure and epithelial junctional proteins were analyzed. After ischemia, there was increased transmural permeability, loss of villi structure, and destruction of epithelial proteins. Supplementation with luminal glucose preserved the epithelium and significantly attenuated permeability and villi damage. Matrix metalloproteinase (MMP) inhibitors (doxycycline, GM 6001), and serine protease inhibitor (tranexamic acid) in the lumen, significantly reduced the fluorescein transport compared to saline for 90 min of ischemia. Based on these results, we tested in an in-vivo model of hemorrhagic shock (90 min 30 mmHg, 3 hours observation) for intestinal lesion formation. Single enteral interventions (saline, glucose, tranexamic acid) did not prevent intestinal lesions, while the combination of enteral glucose and tranexamic acid prevented lesion formation after hemorrhagic shock. The results suggest that apoptotic and protease mediated breakdown cause increased permeability and damage to the intestinal wall. Metabolic support in the lumen of an ischemic intestine with glucose reduces the transport from the lumen across the wall and enteral proteolytic inhibition attenuates tissue breakdown. These combined interventions ameliorate lesion formation in the small intestine after hemorrhagic shock.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

