Role of helicity of α-helical antimicrobial peptides to improve specificity
- PMID: 24805306
- PMCID: PMC4130925
- DOI: 10.1007/s13238-014-0061-0
Role of helicity of α-helical antimicrobial peptides to improve specificity
Abstract
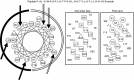
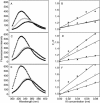
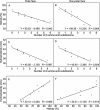
A major barrier to the use of antimicrobial peptides as antibiotics is the toxicity or ability to lyse eukaryotic cells. In this study, a 26-residue amphipathic α-helical antimicrobial peptide A12L/A20L (Ac-KWKSFLKTFKSLKKTVLHTLLKAISS-amide) was used as the framework to design a series of D- and L-diastereomeric peptides and study the relationships of helicity and biological activities of α-helical antimicrobial peptides. Peptide helicity was measured by circular dichroism spectroscopy and demonstrated to correlate with the hydrophobicity of peptides and the numbers of D-amino acid substitutions. Therapeutic index was used to evaluate the selectivity of peptides against prokaryotic cells. By introducing D-amino acids to replace the original L-amino acids on the non-polar face or the polar face of the helix, the hemolytic activity of peptide analogs have been significantly reduced. Compared to the parent peptide, the therapeutic indices were improved of 44-fold and 22-fold against Gram-negative and Gram-positive bacteria, respectively. In addition, D- and L-diastereomeric peptides exhibited lower interaction with zwitterionic eukaryotic membrane and showed the significant membrane damaging effect to bacterial cells. Helicity was proved to play a crucial role on peptide specificity and biological activities. By simply replacing the hydrophobic or the hydrophilic amino acid residues on the non-polar or the polar face of these amphipathic derivatives of the parent peptide with D-amino acids, we demonstrated that this method could have excellent potential for the rational design of antimicrobial peptides with enhanced specificity.
Figures

Similar articles
-
Role of helicity on the anticancer mechanism of action of cationic-helical peptides.Int J Mol Sci. 2012;13(6):6849-6862. doi: 10.3390/ijms13066849. Epub 2012 Jun 5. Int J Mol Sci. 2012. PMID: 22837667 Free PMC article.
-
Rational design of alpha-helical antimicrobial peptides with enhanced activities and specificity/therapeutic index.J Biol Chem. 2005 Apr 1;280(13):12316-29. doi: 10.1074/jbc.M413406200. Epub 2005 Jan 27. J Biol Chem. 2005. PMID: 15677462 Free PMC article.
-
Effects of single D-amino acid substitutions on disruption of beta-sheet structure and hydrophobicity in cyclic 14-residue antimicrobial peptide analogs related to gramicidin S.J Pept Res. 2004 Feb;63(2):69-84. doi: 10.1046/j.1399-3011.2003.00106.x. J Pept Res. 2004. PMID: 15009528 Free PMC article.
-
Investigation of the Role of Hydrophobic Amino Acids on the Structure-Activity Relationship in the Antimicrobial Venom Peptide Ponericin L1.J Membr Biol. 2022 Oct;255(4-5):537-551. doi: 10.1007/s00232-021-00204-y. Epub 2021 Nov 18. J Membr Biol. 2022. PMID: 34792624 Free PMC article. Review.
-
Antibodies as an unlimited source of anti-infective, anti-tumour and immunomodulatory peptides.Sci Prog. 2014;97(Pt 3):215-33. doi: 10.3184/003685014X14049273183515. Sci Prog. 2014. PMID: 25549407 Free PMC article. Review.
Cited by
-
Evaluation of the antiviral activity of new dermaseptin analogs against Zika virus.Biochem Biophys Rep. 2024 Jun 7;39:101747. doi: 10.1016/j.bbrep.2024.101747. eCollection 2024 Sep. Biochem Biophys Rep. 2024. PMID: 38939125 Free PMC article.
-
Novel D-form of hybrid peptide (D-AP19) rapidly kills Acinetobacter baumannii while tolerating proteolytic enzymes.Sci Rep. 2022 Sep 23;12(1):15852. doi: 10.1038/s41598-022-20236-1. Sci Rep. 2022. PMID: 36151303 Free PMC article.
-
Examining the interactions scorpion venom peptides (HP1090, Meucin-13, and Meucin-18) with the receptor binding domain of the coronavirus spike protein to design a mutated therapeutic peptide.J Mol Graph Model. 2021 Sep;107:107952. doi: 10.1016/j.jmgm.2021.107952. Epub 2021 Jun 3. J Mol Graph Model. 2021. PMID: 34119951 Free PMC article.
-
Immunomodulatory Functions of the Human Cathelicidin LL-37 (aa 13-31)-Derived Peptides are Associated with Predicted α-Helical Propensity and Hydrophobic Index.Biomolecules. 2019 Sep 18;9(9):501. doi: 10.3390/biom9090501. Biomolecules. 2019. PMID: 31540479 Free PMC article.
-
Enantiomeric Effect of d-Amino Acid Substitution on the Mechanism of Action of α-Helical Membrane-Active Peptides.Int J Mol Sci. 2017 Dec 27;19(1):67. doi: 10.3390/ijms19010067. Int J Mol Sci. 2017. PMID: 29280948 Free PMC article.
References
-
- Brinckerhoff LH, Kalashnikov VV, Thompson LW, Yamshchikov GV, Pierce RA, Galavotti HS, Engelhard VH, Slingluff CL., Jr Terminal modifications inhibit proteolytic degradation of an immunogenic MART-1(27–35) peptide: implications for peptide vaccines. Int J Cancer. 1999;83:326–334. doi: 10.1002/(SICI)1097-0215(19991029)83:3<326::AID-IJC7>3.0.CO;2-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

