Effects of CD14 macrophages and proinflammatory cytokines on chondrogenesis in osteoarthritic synovium-derived stem cells
- PMID: 24806317
- PMCID: PMC4195476
- DOI: 10.1089/ten.TEA.2013.0656
Effects of CD14 macrophages and proinflammatory cytokines on chondrogenesis in osteoarthritic synovium-derived stem cells
Abstract
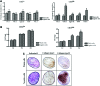
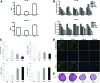
We investigated the effects of CD14 macrophages and proinflammatory cytokines on chondrogenic differentiation of osteoarthritic synovium-derived stem cells (SDSCs). Osteoarthritic synovial fluid was analyzed for interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), and IL-6. Levels of stem cell surface markers in osteoarthritic SDSCs were evaluated using flow cytometry. CD14-negative cells were obtained using magnetically activated cell sorting. We compared chondrogenic potentials between whole cells and CD14-negative cells in CD14(low) cells and CD14(high) cells, respectively. To assess whether nuclear factor-κB (NF-κB) and CCAAT/enhancer-binding protein β (C/EBPβ) modulate IL-1β-induced alterations in chondrogenic potential, we performed small interfering RNA transfection. We observed a significant correlation between the CD14 ratio in osteoarthritic SDSCs and IL-1β and TNF-α in osteoarthritic synovial fluid. Phenotypic characterization of whole cells and CD14-negative cells showed no significant differences in levels of stem cell markers. mRNA expression of type II collagen was higher in CD14-negative cell pellets than in whole cell pellets. Immunohistochemical staining indicated higher levels of type II collagen in the CD14-negative cell pellets of CD14(high) cells than in whole cell pellets of CD14(high) cells. As expected, IL-1β and TNF-α significantly inhibited the expression of chondrogenic-related genes in SDSCs, an effect which was antagonized by knockdown of NF-κB and C/EBPβ. Our results suggest that depletion of CD14(+) synovial macrophages leads to improved chondrogenic potential in CD14(high) cell populations in osteoarthritic SDSCs, and that NF-κB (RelA) and C/EBPβ are critical factors mediating IL-1β-induced suppression of the chondrogenic potential of human SDSCs.
Figures



Similar articles
-
Nerve growth factor regulation and production by macrophages in osteoarthritic synovium.Clin Exp Immunol. 2017 Nov;190(2):235-243. doi: 10.1111/cei.13007. Epub 2017 Jul 27. Clin Exp Immunol. 2017. PMID: 28677145 Free PMC article.
-
Responses to the proinflammatory cytokines interleukin-1 and tumor necrosis factor alpha in cells derived from rheumatoid synovium and other joint tissues involve nuclear factor kappaB-mediated induction of the Ets transcription factor ESE-1.Arthritis Rheum. 2003 May;48(5):1249-60. doi: 10.1002/art.10942. Arthritis Rheum. 2003. PMID: 12746898
-
The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis.Arthritis Res Ther. 2006;8(6):R187. doi: 10.1186/ar2099. Arthritis Res Ther. 2006. PMID: 17177994 Free PMC article.
-
The role of cytokines in osteoarthritis pathophysiology.Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
-
Osteoarthritis joint pain: the cytokine connection.Cytokine. 2014 Dec;70(2):185-93. doi: 10.1016/j.cyto.2014.06.019. Epub 2014 Jul 24. Cytokine. 2014. PMID: 25066335 Free PMC article. Review.
Cited by
-
Mesenchymal Stem Cells for Cartilage Regeneration of TMJ Osteoarthritis.Stem Cells Int. 2017;2017:5979741. doi: 10.1155/2017/5979741. Epub 2017 Oct 16. Stem Cells Int. 2017. PMID: 29123550 Free PMC article. Review.
-
Targeted apoptosis of macrophages and osteoclasts in arthritic joints is effective against advanced inflammatory arthritis.Nat Commun. 2021 Apr 12;12(1):2174. doi: 10.1038/s41467-021-22454-z. Nat Commun. 2021. PMID: 33846342 Free PMC article.
-
Magnesium enhances the chondrogenic differentiation of mesenchymal stem cells by inhibiting activated macrophage-induced inflammation.Sci Rep. 2018 Feb 21;8(1):3406. doi: 10.1038/s41598-018-21783-2. Sci Rep. 2018. PMID: 29467509 Free PMC article.
-
The effect of bone inhibitors on periosteum-guided cartilage regeneration.Sci Rep. 2020 May 20;10(1):8372. doi: 10.1038/s41598-020-65448-5. Sci Rep. 2020. PMID: 32433520 Free PMC article.
-
Therapeutic Manipulation of Macrophages Using Nanotechnological Approaches for the Treatment of Osteoarthritis.Nanomaterials (Basel). 2020 Aug 9;10(8):1562. doi: 10.3390/nano10081562. Nanomaterials (Basel). 2020. PMID: 32784839 Free PMC article. Review.
References
-
- Bedi A., Feeley B.T., and Williams R.J., 3rd Management of articular cartilage defects of the knee. J Bone Joint Surg Am 92,994, 2010 - PubMed
-
- Richardson S.M., Hoyland J.A., Mobasheri R., Csaki C., Shakibaei M., and Mobasheri A.Mesenchymal stem cells in regenerative medicine: opportunities and challenges for articular cartilage and intervertebral disc tissue engineering. J Cell Physiol 222,23, 2010 - PubMed
-
- Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., and Marshak D.R.Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 - PubMed
-
- De Bari C., Dell'Accio F., Tylzanowski P., and Luyten F.P.Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum 44,1928, 2001 - PubMed
-
- Sakaguchi Y., Sekiya I., Yagishita K., and Muneta T.Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum 52,2521, 2005 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

