Alpha-bulges in G protein-coupled receptors
- PMID: 24806342
- PMCID: PMC4057707
- DOI: 10.3390/ijms15057841
Alpha-bulges in G protein-coupled receptors
Abstract
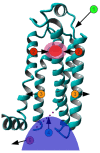
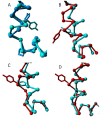
Agonist binding is related to a series of motions in G protein-coupled receptors (GPCRs) that result in the separation of transmembrane helices III and VI at their cytosolic ends and subsequent G protein binding. A large number of smaller motions also seem to be associated with activation. Most helices in GPCRs are highly irregular and often contain kinks, with extensive literature already available about the role of prolines in kink formation and the precise function of these kinks. GPCR transmembrane helices also contain many α-bulges. In this article we aim to draw attention to the role of these α-bulges in ligand and G-protein binding, as well as their role in several aspects of the mobility associated with GPCR activation. This mobility includes regularization and translation of helix III in the extracellular direction, a rotation of the entire helix VI, an inward movement of the helices near the extracellular side, and a concerted motion of the cytosolic ends of the helices that makes their orientation appear more circular and that opens up space for the G protein to bind. In several cases, α-bulges either appear or disappear as part of the activation process.
Figures





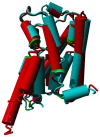
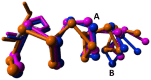
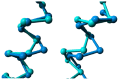
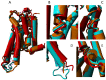


References
-
- PubMed. [accessed on 19 April 2014]. Available online: http://www.ncbi.nlm.nih.gov/pubmed.
-
- Palczewski K., Kumasaka T., Hori T., Behnke C.A., Motoshima H., Fox B.A., le Trong I., Teller D.C., Okada T., Stenkamp R.E., et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–745. - PubMed
-
- GPCR Network. [accessed on 19 April 2014]. Available online: http://gpcr.scripps.edu/
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

