Protein kinase D1 (PKD1) phosphorylation promotes dopaminergic neuronal survival during 6-OHDA-induced oxidative stress
- PMID: 24806360
- PMCID: PMC4013052
- DOI: 10.1371/journal.pone.0096947
Protein kinase D1 (PKD1) phosphorylation promotes dopaminergic neuronal survival during 6-OHDA-induced oxidative stress
Abstract
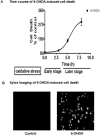
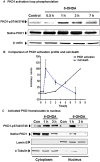
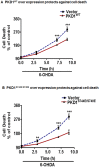
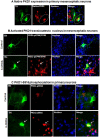
Oxidative stress is a major pathophysiological mediator of degenerative processes in many neurodegenerative diseases including Parkinson's disease (PD). Aberrant cell signaling governed by protein phosphorylation has been linked to oxidative damage of dopaminergic neurons in PD. Although several studies have associated activation of certain protein kinases with apoptotic cell death in PD, very little is known about protein kinase regulation of cell survival and protection against oxidative damage and degeneration in dopaminergic neurons. Here, we characterized the PKD1-mediated protective pathway against oxidative damage in cell culture models of PD. Dopaminergic neurotoxicant 6-hydroxy dopamine (6-OHDA) was used to induce oxidative stress in the N27 dopaminergic cell model and in primary mesencephalic neurons. Our results indicated that 6-OHDA induced the PKD1 activation loop (PKD1S744/S748) phosphorylation during early stages of oxidative stress and that PKD1 activation preceded cell death. We also found that 6-OHDA rapidly increased phosphorylation of the C-terminal S916 in PKD1, which is required for PKD1 activation loop (PKD1S744/748) phosphorylation. Interestingly, negative modulation of PKD1 activation by RNAi knockdown or by the pharmacological inhibition of PKD1 by kbNB-14270 augmented 6-OHDA-induced apoptosis, while positive modulation of PKD1 by the overexpression of full length PKD1 (PKD1WT) or constitutively active PKD1 (PKD1S744E/S748E) attenuated 6-OHDA-induced apoptosis, suggesting an anti-apoptotic role for PKD1 during oxidative neuronal injury. Collectively, our results demonstrate that PKD1 signaling plays a cell survival role during early stages of oxidative stress in dopaminergic neurons and therefore, positive modulation of the PKD1-mediated signal transduction pathway can provide a novel neuroprotective strategy against PD.
Conflict of interest statement
Figures


References
-
- Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302: 819–822. - PubMed
-
- Przedborski S (2005) Pathogenesis of nigral cell death in Parkinson’s disease. Parkinsonism Relat Disord 11 Suppl 1S3–7. - PubMed
-
- Dauer W, Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39: 889–909. - PubMed
-
- Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, et al. (2013) Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: Preclinical and clinical outcomes. Biochim Biophys Acta. http://dx.doi.org/10.1016/j.bbadis.2013.09.007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

