Interleukin-2-stimulated natural killer cells are less susceptible to mycophenolate mofetil than non-activated NK cells: possible consequences for immunotherapy
- PMID: 24806448
- PMCID: PMC11028798
- DOI: 10.1007/s00262-014-1556-5
Interleukin-2-stimulated natural killer cells are less susceptible to mycophenolate mofetil than non-activated NK cells: possible consequences for immunotherapy
Abstract
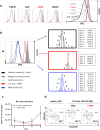
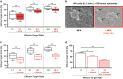
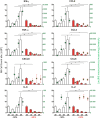
In a clinical phase I/II trial, pediatric patients with high-risk malignancies were treated with ex vivo IL-2-stimulated donor natural killer (NK) cells after transplantation with haploidentical stem cells. To evaluate the potential negative effects of the immunosuppressive drug mycophenolate mofetil (MMF) used for immunotherapy, the functionality and signaling of ex vivo NK cells was investigated. Our results show that during NK cell expansion, long-term (9 days) incubation with mycophenolic acid (MPA), the active metabolite of MMF, in therapeutically relevant concentrations led to the severe inhibition of NK cell proliferation. This correlated with a significantly reduced cytokine/chemokine secretion and the inhibited acquisition of surface receptors regarding cytotoxicity (e.g., NKp30, NKp44, NKp46, NKG2D), adhesion/migration (e.g., ICAM-1/CD54, LFA-1/CD11a, CD62L, CXCR3) and activation (e.g., CD25). Moreover, MPA prevented phosphorylation of the central signaling molecules STAT-3/-4/-5, AKT and ERK1/2. In contrast, short-term (24 h) MPA incubation of IL-2-stimulated NK cells had no or only marginal effects on the activated NK cell phenotype, including receptor expression, cytokine/chemokine secretion and intracellular signaling. Further, short-term MPA incubation only moderately affected the highly cytotoxic activity of previously IL-2-stimulated NK cells. In conclusion, while long-term MPA incubation significantly compromised ex vivo NK cell functionality, previously IL-2-activated NK cells seemed to be rather resistant to short-term MPA treatment. This finding supports the use of IL-2-activated NK cells as immunotherapy, especially for patients treated with MMF after haploidentical stem cell transplantation.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

