The Down syndrome-related protein kinase DYRK1A phosphorylates p27(Kip1) and Cyclin D1 and induces cell cycle exit and neuronal differentiation
- PMID: 24806449
- PMCID: PMC4111700
- DOI: 10.4161/cc.29104
The Down syndrome-related protein kinase DYRK1A phosphorylates p27(Kip1) and Cyclin D1 and induces cell cycle exit and neuronal differentiation
Abstract
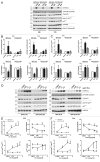
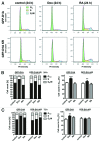
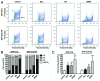
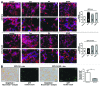
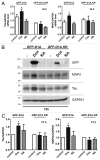
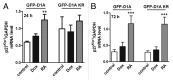
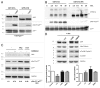
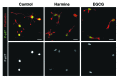
A fundamental question in neurobiology is how the balance between proliferation and differentiation of neuronal precursors is maintained to ensure that the proper number of brain neurons is generated. Substantial evidence implicates DYRK1A (dual specificity tyrosine-phosphorylation-regulated kinase 1A) as a candidate gene responsible for altered neuronal development and brain abnormalities in Down syndrome. Recent findings support the hypothesis that DYRK1A is involved in cell cycle control. Nonetheless, how DYRK1A contributes to neuronal cell cycle regulation and thereby affects neurogenesis remains poorly understood. In the present study we have investigated the mechanisms by which DYRK1A affects cell cycle regulation and neuronal differentiation in a human cell model, mouse neurons, and mouse brain. Dependent on its kinase activity and correlated with the dosage of overexpression, DYRK1A blocked proliferation of SH-SY5Y neuroblastoma cells within 24 h and arrested the cells in G₁ phase. Sustained overexpression of DYRK1A induced G₀ cell cycle exit and neuronal differentiation. Furthermore, we provide evidence that DYRK1A modulated protein stability of cell cycle-regulatory proteins. DYRK1A reduced cellular Cyclin D1 levels by phosphorylation on Thr286, which is known to induce proteasomal degradation. In addition, DYRK1A phosphorylated p27(Kip1) on Ser10, resulting in protein stabilization. Inhibition of DYRK1A kinase activity reduced p27(Kip1) Ser10 phosphorylation in cultured hippocampal neurons and in embryonic mouse brain. In aggregate, these results suggest a novel mechanism by which overexpression of DYRK1A may promote premature neuronal differentiation and contribute to altered brain development in Down syndrome.
Keywords: Cyclin D1; DYRK1A; Down syndrome; cell cycle; neuronal differentiation; p27Kip1; phosphorylation.
Figures

Similar articles
-
Dyrk1A phosphorylates p53 and inhibits proliferation of embryonic neuronal cells.J Biol Chem. 2010 Oct 8;285(41):31895-906. doi: 10.1074/jbc.M110.147520. Epub 2010 Aug 9. J Biol Chem. 2010. PMID: 20696760 Free PMC article.
-
Transient expression of Mnb/Dyrk1a couples cell cycle exit and differentiation of neuronal precursors by inducing p27KIP1 expression and suppressing NOTCH signaling.Development. 2011 Jun;138(12):2543-54. doi: 10.1242/dev.066167. Development. 2011. PMID: 21610031 Free PMC article.
-
Targeting DYRK1A/B kinases to modulate p21-cyclin D1-p27 signalling and induce anti-tumour activity in a model of human glioblastoma.J Cell Mol Med. 2021 Nov;25(22):10650-10662. doi: 10.1111/jcmm.17002. Epub 2021 Oct 28. J Cell Mol Med. 2021. PMID: 34708541 Free PMC article.
-
Cyclin D1 Again Caught in the Act: Dyrk1a Links G1 and Neurogenesis in Down Syndrome.EBioMedicine. 2015 Feb 7;2(2):96-7. doi: 10.1016/j.ebiom.2015.02.003. eCollection 2015. EBioMedicine. 2015. PMID: 26137545 Free PMC article. Review. No abstract available.
-
DYRK1A (dual-specificity tyrosine-phosphorylated and -regulated kinase 1A): a gene with dosage effect during development and neurogenesis.ScientificWorldJournal. 2006 Jun 17;6:1911-22. doi: 10.1100/tsw.2006.319. ScientificWorldJournal. 2006. PMID: 17205196 Free PMC article. Review.
Cited by
-
Emerging proteomic approaches to identify the underlying pathophysiology of neurodevelopmental and neurodegenerative disorders.Mol Autism. 2020 Apr 21;11(1):27. doi: 10.1186/s13229-020-00334-5. Mol Autism. 2020. PMID: 32317014 Free PMC article. Review.
-
Potential Role of Microtubule Stabilizing Agents in Neurodevelopmental Disorders.Int J Mol Sci. 2017 Jul 26;18(8):1627. doi: 10.3390/ijms18081627. Int J Mol Sci. 2017. PMID: 28933765 Free PMC article. Review.
-
Marine-Derived 2-Aminoimidazolone Alkaloids. Leucettamine B-Related Polyandrocarpamines Inhibit Mammalian and Protozoan DYRK & CLK Kinases.Mar Drugs. 2017 Oct 17;15(10):316. doi: 10.3390/md15100316. Mar Drugs. 2017. PMID: 29039762 Free PMC article. Review.
-
Identification of DYRK1B as a substrate of ERK1/2 and characterisation of the kinase activity of DYRK1B mutants from cancer and metabolic syndrome.Cell Mol Life Sci. 2016 Feb;73(4):883-900. doi: 10.1007/s00018-015-2032-x. Epub 2015 Sep 7. Cell Mol Life Sci. 2016. PMID: 26346493 Free PMC article.
-
SDF-1/CXCR4 Axis Regulates Cell Cycle Progression and Epithelial-Mesenchymal Transition via Up-regulation of Survivin in Glioblastoma.Mol Neurobiol. 2016 Jan;53(1):210-215. doi: 10.1007/s12035-014-9006-0. Epub 2014 Nov 25. Mol Neurobiol. 2016. Retraction in: Mol Neurobiol. 2017 Nov;54(9):7550. doi: 10.1007/s12035-017-0508-4. PMID: 25421212 Retracted.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
