Improving the secretion of a methyl parathion hydrolase in Pichia pastoris by modifying its N-terminal sequence
- PMID: 24806460
- PMCID: PMC4013123
- DOI: 10.1371/journal.pone.0096974
Improving the secretion of a methyl parathion hydrolase in Pichia pastoris by modifying its N-terminal sequence
Abstract
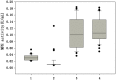
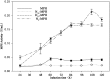
Pichia pastoris is commonly used to express and secrete target proteins, although not all recombinant proteins can be successfully produced. In this study, we used methyl parathion hydrolase (MPH) from Ochrobactrum sp. M231 as a model to study the importance of the N-terminus of the protein for its secretion. While MPH can be efficiently expressed intracellularly in P. pastoris, it is not secreted into the extracellular environment. Three MPH mutants (N66-MPH, D10-MPH, and N9-MPH) were constructed through modification of its N-terminus, and the secretion of each by P. pastoris was improved when compared to wild-type MPH. The level of secreted D10-MPH was increased to 0.21 U/mL, while that of N9-MPH was enhanced to 0.16 U/mL. Although N66-MPH was not enzymatically active, it was secreted efficiently, and was identified by SDS-PAGE. These results demonstrate that the secretion of heterologous proteins in P. pastoris may be improved by modifying their N-terminal structures.
Conflict of interest statement
Figures



References
-
- Higgins DR, Cregg JM (1998) Introduction to Pichia pastoris . Methods Mol Biol 103: 1–15. - PubMed
-
- Cereghino JL, Cregg JM (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris . FEMS Microbiol Rev 24: 45–66. - PubMed
-
- Inan M, Fanders SA, Zhang W, Hotez PJ, Zhan B, et al. (2007) Saturation of the secretory pathway by overexpression of a hookworm (Necator americanus) Protein (Na-ASP1). Methods Mol Biol 389: 65–76. - PubMed
-
- Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM (2005) Heterologous protein production using the Pichia pastoris expression system. Yeast 22: 249–270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

