Genetic analysis reveals that amyloid precursor protein and death receptor 6 function in the same pathway to control axonal pruning independent of β-secretase
- PMID: 24806670
- PMCID: PMC4012303
- DOI: 10.1523/JNEUROSCI.3522-13.2014
Genetic analysis reveals that amyloid precursor protein and death receptor 6 function in the same pathway to control axonal pruning independent of β-secretase
Abstract
In the developing brain, initial neuronal projections are formed through extensive growth and branching of developing axons, but many branches are later pruned to sculpt the mature pattern of connections. Despite its widespread occurrence, the mechanisms controlling pruning remain incompletely characterized. Based on pharmacological and biochemical analysis in vitro and initial genetic analysis in vivo, prior studies implicated a pathway involving binding of the Amyloid Precursor Protein (APP) to Death Receptor 6 (DR6) and activation of a downstream caspase cascade in axonal pruning. Here, we further test their involvement in pruning in vivo and their mechanism of action through extensive genetic and biochemical analysis. Genetic deletion of DR6 was previously shown to impair pruning of retinal axons in vivo. We show that genetic deletion of APP similarly impairs pruning of retinal axons in vivo and provide evidence that APP and DR6 act cell autonomously and in the same pathway to control pruning. Prior analysis had suggested that β-secretase cleavage of APP and binding of an N-terminal fragment of APP to DR6 is required for their actions, but further genetic and biochemical analysis reveals that β-secretase activity is not required and that high-affinity binding to DR6 requires a more C-terminal portion of the APP ectodomain. These results provide direct support for the model that APP and DR6 function cell autonomously and in the same pathway to control pruning in vivo and raise the possibility of alternate mechanisms for how APP and DR6 control pruning.
Keywords: degeneration; pruning.
Figures

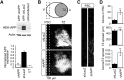
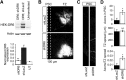

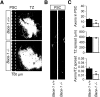


References
-
- Bittner T, Fuhrmann M, Burgold S, Jung CK, Volbracht C, Steiner H, Mitteregger G, Kretzschmar HA, Haass C, Herms J. Gamma-secretase inhibition reduces spine density in vivo via an amyloid precursor protein-dependent pathway. J Neurosci. 2009;29:10405–10409. doi: 10.1523/JNEUROSCI.2288-09.2009. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
