β-Catenin is required for hair-cell differentiation in the cochlea
- PMID: 24806673
- PMCID: PMC4012306
- DOI: 10.1523/JNEUROSCI.4305-13.2014
β-Catenin is required for hair-cell differentiation in the cochlea
Abstract
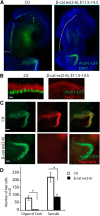
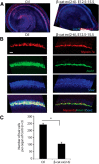
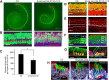
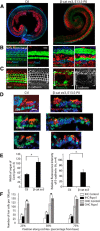
The development of hair cells in the auditory system can be separated into steps; first, the establishment of progenitors for the sensory epithelium, and second, the differentiation of hair cells. Although the differentiation of hair cells is known to require the expression of basic helix-loop-helix transcription factor, Atoh1, the control of cell proliferation in the region of the developing cochlea that will ultimately become the sensory epithelium and the cues that initiate Atoh1 expression remain obscure. We assessed the role of Wnt/β-catenin in both steps in gain- and loss-of-function models in mice. The canonical Wnt pathway mediator, β-catenin, controls the expression of Atoh1. Knock-out of β-catenin inhibited hair-cell, as well as pillar-cell, differentiation from sensory progenitors but was not required to maintain a hair-cell fate once specified. Constitutive activation of β-catenin expanded sensory progenitors by inducing additional cell division and resulted in the differentiation of extra hair cells. Our data demonstrate that β-catenin plays a role in cell division and differentiation in the cochlear sensory epithelium.
Figures




References
-
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
