GABAB agonism promotes sleep and reduces cataplexy in murine narcolepsy
- PMID: 24806675
- PMCID: PMC4012308
- DOI: 10.1523/JNEUROSCI.0080-14.2014
GABAB agonism promotes sleep and reduces cataplexy in murine narcolepsy
Abstract
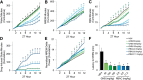
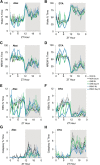
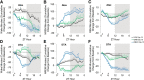
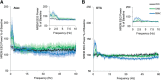
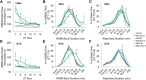
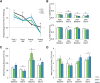
γ-Hydroxybutyrate (GHB) is an approved therapeutic for the excessive sleepiness and sudden loss of muscle tone (cataplexy) characteristic of narcolepsy. The mechanism of action for these therapeutic effects is hypothesized to be GABAB receptor dependent. We evaluated the effects of chronic administration of GHB and the GABAB agonist R-baclofen (R-BAC) on arousal state and cataplexy in two models of narcolepsy: orexin/ataxin-3 (Atax) and orexin/tTA; TetO diphtheria toxin mice (DTA). Mice were implanted for EEG/EMG monitoring and dosed with GHB (150 mg/kg), R-BAC (2.8 mg/kg), or vehicle (VEH) bid for 15 d-a treatment paradigm designed to model the twice nightly GHB dosing regimen used by human narcoleptics. In both models, R-BAC increased NREM sleep time, intensity, and consolidation during the light period; wake bout duration increased and cataplexy decreased during the subsequent dark period. GHB did not increase NREM sleep consolidation or duration, although NREM delta power increased in the first hour after dosing. Cataplexy decreased from baseline in 57 and 86% of mice after GHB and R-BAC, respectively, whereas cataplexy increased in 79% of the mice after VEH. At the doses tested, R-BAC suppressed cataplexy to a greater extent than GHB. These results suggest utility of R-BAC-based therapeutics for narcolepsy.
Keywords: GABA; cataplexy; hypocretin; narcolepsy; orexin; therapeutics.
Figures


Similar articles
-
Almorexant promotes sleep and exacerbates cataplexy in a murine model of narcolepsy.Sleep. 2013 Mar 1;36(3):325-36. doi: 10.5665/sleep.2442. Sleep. 2013. PMID: 23449602 Free PMC article.
-
Conditional ablation of orexin/hypocretin neurons: a new mouse model for the study of narcolepsy and orexin system function.J Neurosci. 2014 May 7;34(19):6495-509. doi: 10.1523/JNEUROSCI.0073-14.2014. J Neurosci. 2014. PMID: 24806676 Free PMC article.
-
Narcolepsy: action of two gamma-aminobutyric acid type B agonists, baclofen and sodium oxybate.Pediatr Neurol. 2009 Jul;41(1):9-16. doi: 10.1016/j.pediatrneurol.2009.02.008. Pediatr Neurol. 2009. PMID: 19520267 Clinical Trial.
-
Emerging drugs for narcolepsy.Expert Opin Emerg Drugs. 2004 Nov;9(2):281-91. doi: 10.1517/14728214.9.2.281. Expert Opin Emerg Drugs. 2004. PMID: 15571485 Review.
-
[Narcolepsy: a new perspective on diagnosis and treatment].Ned Tijdschr Geneeskd. 2007 Apr 14;151(15):856-61. Ned Tijdschr Geneeskd. 2007. PMID: 17472116 Review. Dutch.
Cited by
-
The effect of hydro-alcoholic of Phoenix Dactylifera extract on sleep and EEG in rat.Avicenna J Phytomed. 2017 Nov-Dec;7(6):511-518. Avicenna J Phytomed. 2017. PMID: 29299434 Free PMC article.
-
Current and Future Treatment Options for Narcolepsy: A Review.Sleep Sci. 2017 Jan-Mar;10(1):19-27. doi: 10.5935/1984-0063.20170004. Sleep Sci. 2017. PMID: 28966734 Free PMC article.
-
Trace Amine-Associated Receptor 1 Agonists as Narcolepsy Therapeutics.Biol Psychiatry. 2017 Nov 1;82(9):623-633. doi: 10.1016/j.biopsych.2016.10.012. Epub 2016 Oct 18. Biol Psychiatry. 2017. PMID: 27919403 Free PMC article.
-
The mystery of gamma-hydoxybutyrate efficacy in narcolepsy type 1.Sleep. 2023 Sep 8;46(9):zsad156. doi: 10.1093/sleep/zsad156. Sleep. 2023. PMID: 37260387 Free PMC article. No abstract available.
-
Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models.Proc Natl Acad Sci U S A. 2017 May 30;114(22):5731-5736. doi: 10.1073/pnas.1700499114. Epub 2017 May 15. Proc Natl Acad Sci U S A. 2017. PMID: 28507129 Free PMC article.
References
-
- Absalom N, Eghorn LF, Villumsen IS, Karim N, Bay T, Olsen JV, Knudsen GM, Bräuner-Osborne H, Frølund B, Clausen RP, Chebib M, Wellendorph P. α4βδ GABAA receptors are high-affinity targets for γ-hydroxybutyric acid (GHB) Proc Natl Acad Sci U S A. 2012;109:13404–13409. doi: 10.1073/pnas.1204376109. - DOI - PMC - PubMed
-
- Berry-Kravis EM, Hessl D, Rathmell B, Zarevics P, Cherubini M, Walton-Bowen K, Mu Y, Nguyen DV, Gonzalez-Heydrich J, Wang PP, Carpenter RL, Bear MF, Hagerman RJ. Effects of STX209 (arbaclofen) on neurobehavioral function in children and adults with fragile X syndrome: a randomized, controlled, phase 2 trial. Sci Transl Med. 2012;4:152ra127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
