Modulation of C. elegans touch sensitivity is integrated at multiple levels
- PMID: 24806678
- PMCID: PMC4012311
- DOI: 10.1523/JNEUROSCI.0022-14.2014
Modulation of C. elegans touch sensitivity is integrated at multiple levels
Abstract
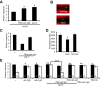
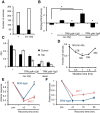
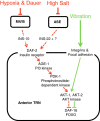
Sensory systems can adapt to different environmental signals. Here we identify four conditions that modulate anterior touch sensitivity in Caenorhabditis elegans after several hours and demonstrate that such sensory modulation is integrated at multiple levels to produce a single output. Prolonged vibration involving integrin signaling directly sensitizes the touch receptor neurons (TRNs). In contrast, hypoxia, the dauer state, and high salt reduce touch sensitivity by preventing the release of long-range neuroregulators, including two insulin-like proteins. Integration of these latter inputs occurs at upstream neurohormonal cells and at the insulin signaling cascade within the TRNs. These signals and those from integrin signaling converge to modulate touch sensitivity by regulating AKT kinases and DAF-16/FOXO. Thus, activation of either the integrin or insulin pathways can compensate for defects in the other pathway. This modulatory system integrates conflicting signals from different modalities, and adapts touch sensitivity to both mechanical and non-mechanical conditions.
Keywords: insulin signaling; integrin signaling; long-term sensitization; mechanosensation; sensory modulation.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
