Cyclic AMP and afferent activity govern bidirectional synaptic plasticity in striatopallidal neurons
- PMID: 24806695
- PMCID: PMC4012319
- DOI: 10.1523/JNEUROSCI.3906-13.2014
Cyclic AMP and afferent activity govern bidirectional synaptic plasticity in striatopallidal neurons
Abstract
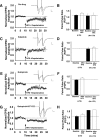
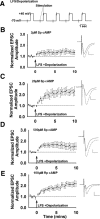
Recent experimental evidence suggests that the low dopamine conditions in Parkinson's disease (PD) cause motor impairment through aberrant motor learning. Those data, along with computational models, suggest that this aberrant learning results from maladaptive corticostriatal plasticity and learned motor inhibition. Dopaminergic modulation of both corticostriatal long-term depression (LTD) and long-term potentiation (LTP) is proposed to be critical for these processes; however, the regulatory mechanisms underlying bidirectional corticostriatal plasticity are not fully understood. Previously, we demonstrated a key role for cAMP signaling in corticostriatal LTD. In this study, mouse brain slices were used to perform a parametric experiment that tested the impact of varying both intracellular cAMP levels and the strength of excitatory inputs on corticostriatal plasticity. Using slice electrophysiology in the dorsolateral striatum, we demonstrate that both LTP and LTD can be sequentially induced in the same D2-expressing neuron and that LTP was strongest with high intracellular cAMP and LFS, whereas LTD required low intracellular cAMP and high-frequency stimulation. Our results provide a molecular and cellular basis for regulating bidirectional corticostriatal synaptic plasticity and may help to identify novel therapeutic targets for blocking or reversing the aberrant synaptic plasticity that likely contributes to motor deficits in PD.
Keywords: LTD; LTP; cAMP; dorsolateral striatum; motor learning; synaptic plasticity.
Figures



References
-
- Bagetta V, Picconi B, Marinucci S, Sgobio C, Pendolino V, Ghiglieri V, Fusco FR, Giampà C, Calabresi P. Dopamine-dependent long-term depression is expressed in striatal spiny neurons of both direct and indirect pathways: implications for Parkinson's disease. J Neurosci. 2011;31:12513–12522. doi: 10.1523/JNEUROSCI.2236-11.2011. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
