Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma
- PMID: 24807677
- PMCID: PMC4186664
- DOI: 10.1111/php.12286
Combination of oral vitamin D3 with photodynamic therapy enhances tumor cell death in a murine model of cutaneous squamous cell carcinoma
Abstract
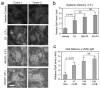
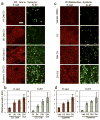
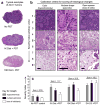
Photodynamic therapy (PDT), in which 5-ALA (a precursor for protoporphyrin IX, PpIX) is administered prior to exposure to light, is a nonscarring treatment for skin cancers. However, for deep tumors, ALA-PDT is not always effective due to inadequate production of PpIX. We previously developed and reported a combination approach in which the active form of vitamin D3 (calcitriol) is given systemically prior to PDT to improve PpIX accumulation and to enhance PDT-induced tumor cell death; calcitriol, however, poses a risk of hypercalcemia. Here, we tested a possible strategy to circumvent the problem of hypercalcemia by substituting natural dietary vitamin D3 (cholecalciferol; D3 ) for calcitriol. Oral D3 supplementation (10 days of a 10-fold elevated D3 diet) enhanced PpIX levels 3- to 4-fold, and PDT-mediated cell death 20-fold, in subcutaneous A431 tumors. PpIX levels and cell viability in normal tissues were not affected. Hydroxylated metabolic forms of D3 were only modestly elevated in serum, indicating minimal hypercalcemic risk. These results show that brief oral administration of cholecalciferol can serve as a safe neoadjuvant to ALA-PDT. We suggest a clinical study, using oral vitamin D3 prior to PDT, should be considered to evaluate this promising new approach to treating human skin cancer.
© 2014 The American Society of Photobiology.
Figures


References
-
- Rogers HW, Weinstock MA, Harris AR, Hinckley MR, Feldman SR, Fleischer B, Coldiron BM. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. - PubMed
-
- Donaldson MR, Coldiron BM. No end in sight: the skin cancer epidemic continues. Semin Cutan Med Surg. 2011;30:3–5. - PubMed
-
- Hasan T, Ortel B, Solban N, Pogue BW. Photodynamic therapy of cancer. In: Kufe D, Bast R, Hait W, Hong W, Pollock R, Weichselbaum R, Holland J, Frei E, editors. Cancer Medicine. 7. BC Decker, Inc; Hamilton, ON: 2006. pp. 537–548.
-
- Ortel B, Shea CR, Calzavara-Pinton P. Molecular mechanisms of photodynamic therapy. Front Biosci. 2009;14:4157–4172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

