Novel roles of cytoplasmic ICP0: proteasome-independent functions of the RING finger are required to block interferon-stimulated gene production but not to promote viral replication
- PMID: 24807717
- PMCID: PMC4097794
- DOI: 10.1128/JVI.00944-14
Novel roles of cytoplasmic ICP0: proteasome-independent functions of the RING finger are required to block interferon-stimulated gene production but not to promote viral replication
Abstract
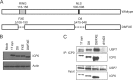
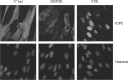
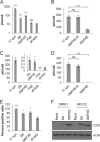
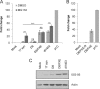
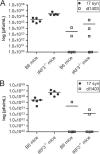
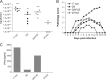
The immediate-early protein ICP0 from herpes simplex virus 1 (HSV-1) plays pleiotropic roles in promoting viral lytic replication and reactivation from latency. Most of the known actions of ICP0 occur in the nucleus and are thought to involve the E3 ubiquitin ligase activity of its RING finger domain, which targets proteins for degradation via the proteasome. Although ICP0 translocates to the cytoplasm as the infection progresses, little is known about its activities in this location. Here, we show that cytoplasmic ICP0 has two distinct functions. In primary cell cultures and in an intravaginal mouse model, cytoplasmic ICP0 promotes viral replication in the absence of an intact RING finger domain. Additionally, ICP0 blocks the activation of interferon regulatory factor 3 (IRF3), a key transcription factor of the innate antiviral response, in a mechanism that requires the RING finger domain but not the proteasome. To our knowledge, this is the first observation of a proteasome-independent function of the RING finger domain of ICP0. Collectively, these results underscore the importance of cytoplasm-localized ICP0 and the diverse nature of its activities. Importance: Despite ICP0 being a well-studied viral protein, the significance of its cytoplasmic localization has been largely overlooked. This is, in part, because common experimental manipulations result in the restriction of ICP0 to the nucleus. By overcoming this constraint, we both further characterize the ability of cytoplasmic ICP0 to inhibit antiviral signaling and show that ICP0 at this site has unexpected activities in promoting viral replication. This demonstrates the importance of considering location when analyzing protein function and adds a new perspective to our understanding of this multifaceted protein.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

