HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities
- PMID: 24807721
- PMCID: PMC4097802
- DOI: 10.1128/JVI.00156-14
HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities
Abstract
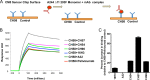
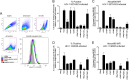
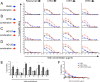
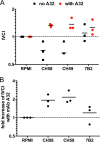
The RV144 ALVAC/AIDSVax HIV-1 vaccine clinical trial showed an estimated vaccine efficacy of 31.2%. Viral genetic analysis identified a vaccine-induced site of immune pressure in the HIV-1 envelope (Env) variable region 2 (V2) focused on residue 169, which is included in the epitope recognized by vaccinee-derived V2 monoclonal antibodies. The ALVAC/AIDSVax vaccine induced antibody-dependent cellular cytotoxicity (ADCC) against the Env V2 and constant 1 (C1) regions. In the presence of low IgA Env antibody levels, plasma levels of ADCC activity correlated with lower risk of infection. In this study, we demonstrate that C1 and V2 monoclonal antibodies isolated from RV144 vaccinees synergized for neutralization, infectious virus capture, and ADCC. Importantly, synergy increased the HIV-1 ADCC activity of V2 monoclonal antibody CH58 at concentrations similar to that observed in plasma of RV144 vaccinees. These findings raise the hypothesis that synergy among vaccine-induced antibodies with different epitope specificities contributes to HIV-1 antiviral antibody responses and is important to induce for reduction in the risk of HIV-1 transmission. Importance: The Thai RV144 ALVAC/AIDSVax prime-boost vaccine efficacy trial represents the only example of HIV-1 vaccine efficacy in humans to date. Studies aimed at identifying immune correlates involved in the modest vaccine-mediated protection identified HIV-1 envelope (Env) variable region 2-binding antibodies as inversely correlated with infection risk, and genetic analysis identified a site of immune pressure within the region recognized by these antibodies. Despite this evidence, the antiviral mechanisms by which variable region 2-specific antibodies may have contributed to lower rates of infection remain unclear. In this study, we demonstrate that vaccine-induced HIV-1 envelope variable region 2 and constant region 1 antibodies synergize for recognition of virus-infected cells, infectious virion capture, virus neutralization, and antibody-dependent cellular cytotoxicity. This is a major step in understanding how these types of antibodies may have cooperatively contributed to reducing infection risk and should be considered in the context of prospective vaccine design.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Recognition Patterns of the C1/C2 Epitopes Involved in Fc-Mediated Response in HIV-1 Natural Infection and the RV114 Vaccine Trial.mBio. 2020 Jun 30;11(3):e00208-20. doi: 10.1128/mBio.00208-20. mBio. 2020. PMID: 32605979 Free PMC article.
-
HIV vaccine delayed boosting increases Env variable region 2-specific antibody effector functions.JCI Insight. 2020 Jan 30;5(2):e131437. doi: 10.1172/jci.insight.131437. JCI Insight. 2020. PMID: 31996483 Free PMC article.
-
Boosting with AIDSVAX B/E Enhances Env Constant Region 1 and 2 Antibody-Dependent Cellular Cytotoxicity Breadth and Potency.J Virol. 2020 Jan 31;94(4):e01120-19. doi: 10.1128/JVI.01120-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31776278 Free PMC article. Clinical Trial.
-
Nonneutralizing functional antibodies: a new "old" paradigm for HIV vaccines.Clin Vaccine Immunol. 2014 Aug;21(8):1023-36. doi: 10.1128/CVI.00230-14. Epub 2014 Jun 11. Clin Vaccine Immunol. 2014. PMID: 24920599 Free PMC article. Review.
-
Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection.Annu Rev Med. 2015;66:423-37. doi: 10.1146/annurev-med-052912-123749. Epub 2014 Oct 17. Annu Rev Med. 2015. PMID: 25341006 Review.
Cited by
-
Defining rules governing recognition and Fc-mediated effector functions to the HIV-1 co-receptor binding site.BMC Biol. 2020 Jul 21;18(1):91. doi: 10.1186/s12915-020-00819-y. BMC Biol. 2020. PMID: 32693837 Free PMC article.
-
HIV-1 Envelope Glycosylation and the Signal Peptide.Vaccines (Basel). 2021 Feb 19;9(2):176. doi: 10.3390/vaccines9020176. Vaccines (Basel). 2021. PMID: 33669676 Free PMC article. Review.
-
Vaccine-Induced, High-Magnitude HIV Env-Specific Antibodies with Fc-Mediated Effector Functions Are Insufficient to Protect Infant Rhesus Macaques against Oral SHIV Infection.mSphere. 2022 Feb 23;7(1):e0083921. doi: 10.1128/msphere.00839-21. Epub 2022 Feb 23. mSphere. 2022. PMID: 35196125 Free PMC article.
-
Selection of HIV Envelope Strains for Standardized Assessments of Vaccine-Elicited Antibody-Dependent Cellular Cytotoxicity-Mediating Antibodies.J Virol. 2022 Jan 26;96(2):e0164321. doi: 10.1128/JVI.01643-21. Epub 2021 Nov 3. J Virol. 2022. PMID: 34730393 Free PMC article.
-
Boosting of HIV envelope CD4 binding site antibodies with long variable heavy third complementarity determining region in the randomized double blind RV305 HIV-1 vaccine trial.PLoS Pathog. 2017 Feb 24;13(2):e1006182. doi: 10.1371/journal.ppat.1006182. eCollection 2017 Feb. PLoS Pathog. 2017. PMID: 28235027 Free PMC article. Clinical Trial.
References
-
- Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, MOPH-TAVEG Investigators. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209–2220. 10.1056/NEJMoa0908492 - DOI - PubMed
-
- Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao H-X, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N. Engl. J. Med. 366:1275–1286. 10.1056/NEJMoa1113425 - DOI - PMC - PubMed
-
- Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, Decamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O'Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O'Connell RJ, deSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. 2012. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature 490:417–420. 10.1038/nature11519 - DOI - PMC - PubMed
-
- Liao H-X, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang K-K, Chen X, Tsao C-Y, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang Z-Y, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186. 10.1016/j.immuni.2012.11.011 - DOI - PMC - PubMed
-
- Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang K-K, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao C-Y, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao H-X, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J. Virol. 86:11521–11532. 10.1128/JVI.01023-12 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

