Cytosolic PrP can participate in prion-mediated toxicity
- PMID: 24807727
- PMCID: PMC4097767
- DOI: 10.1128/JVI.00732-14
Cytosolic PrP can participate in prion-mediated toxicity
Abstract
Prion diseases are characterized by a conformational change in the normal host protein PrPC. While the majority of mature PrPC is tethered to the plasma membrane by a glycosylphosphatidylinositol anchor, topological variants of this protein can arise during its biosynthesis. Here we have generated Drosophila transgenic for cytosolic ovine PrP in order to investigate its toxic potential in flies in the absence or presence of exogenous ovine prions. While cytosolic ovine PrP expressed in Drosophila was predominantly detergent insoluble and showed resistance to low concentrations of proteinase K, it was not overtly detrimental to the flies. However, Drosophila transgenic for cytosolic PrP expression exposed to classical or atypical scrapie prion inocula showed a faster decrease in locomotor activity than similar flies exposed to scrapie-free material. The susceptibility to classical scrapie inocula could be assessed in Drosophila transgenic for panneuronal expression of cytosolic PrP, whereas susceptibility to atypical scrapie required ubiquitous PrP expression. Significantly, the toxic phenotype induced by ovine scrapie in cytosolic PrP transgenic Drosophila was transmissible to recipient PrP transgenic flies. These data show that while cytosolic PrP expression does not adversely affect Drosophila, this topological PrP variant can participate in the generation of transmissible scrapie-induced toxicity. These observations also show that PrP transgenic Drosophila are susceptible to classical and atypical scrapie prion strains and highlight the utility of this invertebrate host as a model of mammalian prion disease. Importance: During prion diseases, the host protein PrPC converts into an abnormal conformer, PrPSc, a process coupled to the generation of transmissible prions and neurotoxicity. While PrPC is principally a glycosylphosphatidylinositol-anchored membrane protein, the role of topological variants, such as cytosolic PrP, in prion-mediated toxicity and prion formation is undefined. Here we generated Drosophila transgenic for cytosolic PrP expression in order to investigate its toxic potential in the absence or presence of exogenous prions. Cytosolic ovine PrP expressed in Drosophila was not overtly detrimental to the flies. However, cytosolic PrP transgenic Drosophila exposed to ovine scrapie showed a toxic phenotype absent from similar flies exposed to scrapie-free material. Significantly, the scrapie-induced toxic phenotype in cytosolic transgenic Drosophila was transmissible to recipient PrP transgenic flies. These data show that cytosolic PrP can participate in the generation of transmissible prion-induced toxicity and highlight the utility of Drosophila as a model of mammalian prion disease.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures
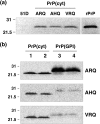
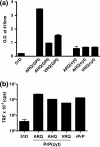
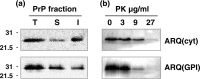


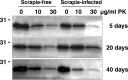



Similar articles
-
Prion-induced and spontaneous formation of transmissible toxicity in PrP transgenic Drosophila.Biochem J. 2014 Oct 1;463(1):31-40. doi: 10.1042/BJ20140129. Biochem J. 2014. PMID: 25000212
-
Prion-induced toxicity in PrP transgenic Drosophila.Exp Mol Pathol. 2012 Apr;92(2):194-201. doi: 10.1016/j.yexmp.2012.01.005. Epub 2012 Jan 31. Exp Mol Pathol. 2012. PMID: 22314254
-
Bioassay of prion-infected blood plasma in PrP transgenic Drosophila.Biochem J. 2016 Dec 1;473(23):4399-4412. doi: 10.1042/BCJ20160417. Epub 2016 Oct 12. Biochem J. 2016. PMID: 27733649
-
Genetic and infectious prion diseases.Arch Neurol. 1993 Nov;50(11):1129-53. doi: 10.1001/archneur.1993.00540110011002. Arch Neurol. 1993. PMID: 8105771 Review.
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
Cited by
-
Transgenic Overexpression of the Disordered Prion Protein N1 Fragment in Mice Does Not Protect Against Neurodegenerative Diseases Due to Impaired ER Translocation.Mol Neurobiol. 2020 Jun;57(6):2812-2829. doi: 10.1007/s12035-020-01917-2. Epub 2020 May 4. Mol Neurobiol. 2020. PMID: 32367491 Free PMC article.
-
Prion-induced neurotoxicity: Possible role for cell cycle activity and DNA damage response.World J Virol. 2015 Aug 12;4(3):188-97. doi: 10.5501/wjv.v4.i3.188. World J Virol. 2015. PMID: 26279981 Free PMC article. Review.
-
Drosophila models of prionopathies: insight into prion protein function, transmission, and neurotoxicity.Curr Opin Genet Dev. 2017 Jun;44:141-148. doi: 10.1016/j.gde.2017.03.013. Epub 2017 Apr 14. Curr Opin Genet Dev. 2017. PMID: 28415023 Free PMC article. Review.
-
Transcriptional signature of prion-induced neurotoxicity in a Drosophila model of transmissible mammalian prion disease.Biochem J. 2020 Feb 28;477(4):833-852. doi: 10.1042/BCJ20190872. Biochem J. 2020. PMID: 32108870 Free PMC article.
-
Prion disease modelled in Drosophila.Cell Tissue Res. 2023 Apr;392(1):47-62. doi: 10.1007/s00441-022-03586-0. Epub 2022 Jan 29. Cell Tissue Res. 2023. PMID: 35092497 Free PMC article. Review.
References
-
- Prusiner SB. (ed). 2004. Prion biology and diseases, second edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

