Effects of different feeder layers on culture of bovine embryonic stem cell-like cells in vitro
- PMID: 24807816
- PMCID: PMC4235940
- DOI: 10.1007/s10616-013-9653-4
Effects of different feeder layers on culture of bovine embryonic stem cell-like cells in vitro
Abstract
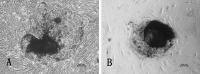
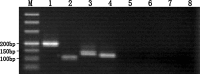
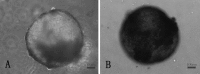
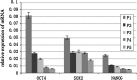
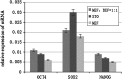
To find a suitable feeder layer is important for successful culture conditions of bovine embryonic stem cell-like cells. In this study, expression of pluripotency-related genes OCT4, SOX2 and NANOG in bovine embryonic stem cell-like cells on mouse embryonic fibroblast feeder layers at 1-5 passages were monitored in order to identify the possible reason that bovine embryonic stem cell-like cells could not continue growth and passage. Here, we developed two novel feeder layers, mixed embryonic fibroblast feeder layers of mouse and bovine embryonic fibroblast at different ratios and sources including mouse fibroblast cell lines. The bovine embryonic stem cell-like cells generated in our study displayed typical stem cell morphology and expressed specific markers such as OCT4, stage-specific embryonic antigen 1 and 4, alkaline phosphatase, SOX2, and NANOG mRNA levels. When feeder layers and cell growth factors were removed, the bovine embryonic stem cell-like cells formed embryoid bodies in a suspension culture. Furthermore, we compared the expression of the pluripotent markers during bovine embryonic stem cell-like cell in culture on mixed embryonic fibroblast feeder layers, including mouse fibroblast cell lines feeder layers and mouse embryonic fibroblast feeder layers by real-time quantitative polymerase chain reaction. Results suggested that mixed embryonic fibroblast and sources including mouse fibroblast cell lines feeder layers were more suitable for long-term culture and growth of bovine embryonic stem cell-like cells than mouse embryonic fibroblast feeder layers. The findings may provide useful experimental data for the establishment of an appropriate culture system for bovine embryonic stem cell lines.
Figures


Similar articles
-
Establishment and growth responses of Nile tilapia embryonic stem-like cell lines under feeder-free condition.Dev Growth Differ. 2017 Feb;59(2):83-93. doi: 10.1111/dgd.12341. Epub 2017 Feb 23. Dev Growth Differ. 2017. PMID: 28230233
-
Preparation of mouse embryonic fibroblast cells suitable for culturing human embryonic and induced pluripotent stem cells.J Vis Exp. 2012 Jun 21;(64):3854. doi: 10.3791/3854. J Vis Exp. 2012. PMID: 22760161 Free PMC article.
-
Establishment of bovine embryonic stem cell lines using a minimized feeder cell drop.Cell Reprogram. 2012 Dec;14(6):520-9. doi: 10.1089/cell.2012.0038. Cell Reprogram. 2012. PMID: 23194455
-
Mesenchymal stem cells as an appropriate feeder layer for prolonged in vitro culture of human induced pluripotent stem cells.Mol Biol Rep. 2013 Apr;40(4):3023-31. doi: 10.1007/s11033-012-2376-3. Epub 2013 Jan 3. Mol Biol Rep. 2013. PMID: 23283738
-
Mouse embryonic stem cell-derived feeder cells support the growth of their own mouse embryonic stem cells.Cell Biol Int. 2006 Dec;30(12):1041-7. doi: 10.1016/j.cellbi.2006.08.003. Epub 2006 Aug 24. Cell Biol Int. 2006. PMID: 17074515
Cited by
-
Bovine Pluripotent Stem Cells: Current Status and Prospects.Int J Mol Sci. 2024 Feb 9;25(4):2120. doi: 10.3390/ijms25042120. Int J Mol Sci. 2024. PMID: 38396797 Free PMC article. Review.
-
Efficient induction and sustenance of pluripotent stem cells from bovine somatic cells.Biol Open. 2021 Oct 15;10(10):bio058756. doi: 10.1242/bio.058756. Epub 2021 Nov 1. Biol Open. 2021. PMID: 34719702 Free PMC article.
-
Mitotically inactivated mosquito cells support robust Wolbachia infection and replication.In Vitro Cell Dev Biol Anim. 2022 Oct;58(9):780-787. doi: 10.1007/s11626-022-00726-2. Epub 2022 Oct 21. In Vitro Cell Dev Biol Anim. 2022. PMID: 36271174
-
Revolutionize livestock breeding in the future: an animal embryo-stem cell breeding system in a dish.J Anim Sci Biotechnol. 2018 Dec 14;9:90. doi: 10.1186/s40104-018-0304-7. eCollection 2018. J Anim Sci Biotechnol. 2018. PMID: 30568797 Free PMC article. Review.
-
Assessment of mitotically inactivated mosquito cell feeder layers produced with mitomycin C.In Vitro Cell Dev Biol Anim. 2021 Jun;57(6):583-586. doi: 10.1007/s11626-021-00597-z. Epub 2021 Jun 28. In Vitro Cell Dev Biol Anim. 2021. PMID: 34184209 No abstract available.
References
-
- Anand T, Kumar D, Singh MK, Shah RA, Chauhan MS, Manik RS, Singla SK, Palta P. Buffalo (Bubalus bubalis) embryonic stem cell-like cells and preimplantation embryos exhibit comparable expression of pluripotency-related antigens. Reprod Domest Anim. 2011;46:50–58. doi: 10.1111/j.1439-0531.2009.01564.x. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

