Emerging functions of SRSF1, splicing factor and oncoprotein, in RNA metabolism and cancer
- PMID: 24807918
- PMCID: PMC4163531
- DOI: 10.1158/1541-7786.MCR-14-0131
Emerging functions of SRSF1, splicing factor and oncoprotein, in RNA metabolism and cancer
Abstract
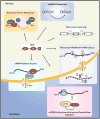
Serine/Arginine Splicing Factor 1 (SRSF1) is the archetype member of the SR protein family of splicing regulators. Since its discovery over two decades ago, SRSF1 has been repeatedly surprising and intriguing investigators by the plethora of complex biologic pathways it regulates. These include several key aspects of mRNA metabolism, such as mRNA splicing, stability, and translation, as well as other mRNA-independent processes, such as miRNA processing, protein sumoylation, and the nucleolar stress response. In this review, the structural features of SRSF1 are discussed as they relate to the intricate mechanism of splicing and the multiplicity of functions it performs. Similarly, a list of relevant alternatively spliced transcripts and SRSF1 interacting proteins is provided. Finally, emphasis is given to the deleterious consequences of overexpression of the SRSF1 proto-oncogene in human cancers, and the complex mechanisms and pathways underlying SRSF1-mediated transformation. The accumulated knowledge about SRSF1 provides critical insight into the integral role it plays in maintaining cellular homeostasis and suggests new targets for anticancer therapy. Mol Cancer Res; 12(9); 1195-204. ©2014 AACR.
©2014 American Association for Cancer Research.
Conflict of interest statement
No potential conflicts of interests were disclosed.
Figures

References
-
- Long JC, Cáceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 2009;417:15–27. - PubMed
-
- Cowper AE, Cáceres JF, Mayeda A, Screaton GR. Serine-arginine (SR) protein-like factors that antagonize authentic SR proteins and regulate alternative splicing. J Biol Chem. 2001;276:48908–14. - PubMed
-
- Sapra AK, Ankö ML, Grishina I, Lorenz M, Pabis M, Poser I, et al. SR protein family members display diverse activities in the formation of nascent and mature mRNPs in vivo. Mol Cell. 2009;24:179–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

