Na+ interactions with the neutral amino acid transporter ASCT1
- PMID: 24808181
- PMCID: PMC4067185
- DOI: 10.1074/jbc.M114.565242
Na+ interactions with the neutral amino acid transporter ASCT1
Abstract
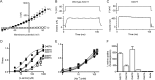
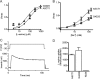
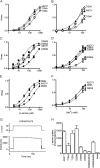
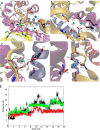
The alanine, serine, cysteine transporters (ASCTs) belong to the solute carrier family 1A (SLC1A), which also includes the excitatory amino acid transporters (EAATs) and the prokaryotic aspartate transporter GltPh. Acidic amino acid transport by the EAATs is coupled to the co-transport of three Na(+) ions and one proton, and the counter-transport of one K(+) ion. In contrast, neutral amino acid exchange by the ASCTs does not require protons or the counter-transport of K(+) ions and the number of Na(+) ions required is not well established. One property common to SLC1A family members is a substrate-activated anion conductance. We have investigated the number and location of Na(+) ions required by ASCT1 by mutating residues in ASCT1 that correspond to residues in the EAATs and GltPh that are involved in Na(+) binding. Mutations to all three proposed Na(+) sites influence the binding of substrate and/or Na(+), or the rate of substrate exchange. A G422S mutation near the Na2 site reduced Na(+) affinity, without affecting the rate of exchange. D467T and D467A mutations in the Na1 site reduce Na(+) and substrate affinity and also the rate of substrate exchange. T124A and D380A mutations in the Na3 site selectively reduce the affinity for Na(+) and the rate of substrate exchange without affecting substrate affinity. In many of the mutants that reduce the rate of substrate transport the amplitudes of the substrate-activated anion conductances are not substantially affected indicating altered ion dependence for channel activation compared with substrate exchange.
Keywords: ASCT; Amino Acid Transport; Chloride Channel; EAAT; Electrophysiology; GltPh; Membrane Transport; Na+ Coupling; Site-directed Mutagenesis.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Christensen H. N., Liang M., Archer E. G. (1967) A distinct Na+-requiring transport system for alanine, serine, cysteine, and similar amino acids. J. Biol. Chem. 242, 5237–5246 - PubMed
-
- Christensen H. (1990) Role of amino acid transport and countertransport in nutrition and metabolism. Physiol. Rev. 70, 43–77 - PubMed
-
- Arriza J. L., Kavanaugh M. P., Fairman W. A., Wu Y. N., Murdoch G. H., North R. A., Amara S. G. (1993) Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J. Biol. Chem. 268, 15329–15332 - PubMed
-
- Shafqat S., Tamarappoo B. K., Kilberg M. S., Puranam R. S., McNamara J. O., Guadaño-Ferraz A., Fremeau R. T., Jr. (1993) Cloning and expression of a novel Na+-dependent neutral amino acid transporter structurally related to mammalian Na+/glutamate cotransporters. J. Biol. Chem. 268, 15351–15355 - PubMed
-
- Utsunomiya-Tate N., Endou H., Kanai Y. (1996) Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J. Biol. Chem. 271, 14883–14890 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

