Role of 2',3'-cyclic nucleotide 3'-phosphodiesterase in the renal 2',3'-cAMP-adenosine pathway
- PMID: 24808540
- PMCID: PMC4080157
- DOI: 10.1152/ajprenal.00134.2014
Role of 2',3'-cyclic nucleotide 3'-phosphodiesterase in the renal 2',3'-cAMP-adenosine pathway
Abstract
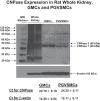
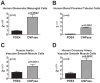
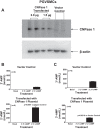
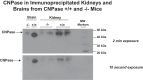
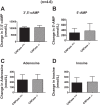
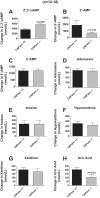
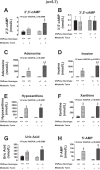
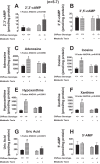
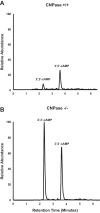
Energy depletion increases the renal production of 2',3'-cAMP (a positional isomer of 3',5'-cAMP that opens mitochondrial permeability transition pores) and 2',3'-cAMP is converted to 2'-AMP and 3'-AMP, which in turn are metabolized to adenosine. Because the enzymes involved in this "2',3'-cAMP-adenosine pathway" are unknown, we examined whether 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNPase) participates in the renal metabolism of 2',3'-cAMP. Western blotting and real-time PCR demonstrated expression of CNPase in rat glomerular mesangial, preglomerular vascular smooth muscle and endothelial, proximal tubular, thick ascending limb and collecting duct cells. Real-time PCR established the expression of CNPase in human glomerular mesangial, proximal tubular and vascular smooth muscle cells; and the level of expression of CNPase was greater than that for phosphodiesterase 4 (major enzyme for the metabolism of 3',5'-cAMP). Overexpression of CNPase in rat preglomerular vascular smooth muscle cells increased the metabolism of exogenous 2',3'-cAMP to 2'-AMP. Infusions of 2',3'-cAMP into isolated CNPase wild-type (+/+) kidneys increased renal venous 2'-AMP, and this response was diminished by 63% in CNPase knockout (-/-) kidneys, whereas the conversion of 3',5'-cAMP to 5'-AMP was similar in CNPase +/+ vs. -/- kidneys. In CNPase +/+ kidneys, energy depletion (metabolic poisons) increased kidney tissue levels of adenosine and its metabolites (inosine, hypoxanthine, xanthine, and uric acid) without accumulation of 2',3'-cAMP. In contrast, in CNPase -/- kidneys, energy depletion increased kidney tissue levels of 2',3'-cAMP and abolished the increase in adenosine and its metabolites. In conclusion, kidneys express CNPase, and renal CNPase mediates in part the renal 2',3'-cAMP-adenosine pathway.
Keywords: 2′,3′-cyclic adenosine monophosphate; 2′,3′-cyclic nucleotide 3′-phosphodiesterase; 2′-adenosine monophosphate; 3′-adenosine monophosphate; CNPase; adenosine.
Copyright © 2014 the American Physiological Society.
Figures

References
-
- Arnoys EJ, Wang JL. Dual localization: proteins in extracellular and intracellular compartments. Acta Histochem 109: 89–110, 2007 - PubMed
-
- Azarashvili T, Krestinina O, Galvita A, Grachev D, Baburina Y, Stricker R, Evtodienko Y, Reiser G. Ca2+-dependent permeability transition regulation in rat brain mitochondria by 2′,3′-cyclic nucleotides and 2′,3′-cyclic nucleotide 3′-phosphodiesterase. Am J Physiol Cell Physiol 296: C1428–C1439, 2009 - PubMed
-
- Bauerle JD, Grenz A, Kim JH, Lee HT, Eltzschig HK. Adenosine generation and signaling during acute kidney injury. J Am Soc Nephrol 22: 14–20, 2011 - PubMed
-
- Darlot F, Artuso A, Lautredou-Audouy N, Casellas D. Topology of Schwann cells and sympathetic innervation along preglomerular vessels: a confocal microscopic study in protein S100B/EGFP transgenic mice. Am J Physiol Renal Physiol 295: F1142–F1148, 2008 - PubMed
-
- Day YJ, Huang L, Ye H, Linden J, Okusa MD. Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: role of macrophages. Am J Physiol Renal Physiol 288: F722–F731, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

