Use of diffusion spectrum imaging in preliminary longitudinal evaluation of amyotrophic lateral sclerosis: development of an imaging biomarker
- PMID: 24808852
- PMCID: PMC4010737
- DOI: 10.3389/fnhum.2014.00270
Use of diffusion spectrum imaging in preliminary longitudinal evaluation of amyotrophic lateral sclerosis: development of an imaging biomarker
Abstract
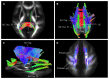
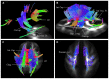
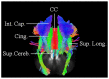
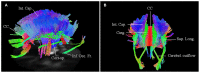
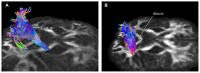
Previous diffusion tensor imaging (DTI) studies have shown white matter pathology in amyotrophic lateral sclerosis (ALS), predominantly in the motor pathways. Further these studies have shown that DTI can be used longitudinally to track pathology over time, making white matter pathology a candidate as an outcome measure in future trials. DTI has demonstrated application in group studies, however its derived indices, for example fractional anisotropy, are susceptible to partial volume effects, making its role questionable in examining individual progression. We hypothesize that changes in the white matter are present in ALS beyond the motor tracts, and that the affected pathways and associated pattern of disease progression can be tracked longitudinally using automated diffusion connectometry analysis. Connectometry analysis is based on diffusion spectrum imaging and overcomes the limitations of a conventional tractography approach and DTI. The identified affected white matter tracts can then be assessed in a targeted fashion using High definition fiber tractography (a novel white matter MR imaging technique). Changes in quantitative and qualitative markers over time could then be correlated with clinical progression. We illustrate these principles toward developing an imaging biomarker for demonstrating individual progression, by presenting results for five ALS patients, including with longitudinal data in two. Preliminary analysis demonstrated a number of changes bilaterally and asymmetrically in motoric and extramotoric white matter pathways. Further the limbic system was also affected possibly explaining the cognitive symptoms in ALS. In the two longitudinal subjects, the white matter changes were less extensive at baseline, although there was evidence of disease progression in a frontal pattern with a relatively spared postcentral gyrus, consistent with the known pathology in ALS.
Keywords: amyotrophic lateral sclerosis; diffusion spectrum imaging; extramotoric; longitudinal; motoric; white matter.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

