Nitric oxide donors for treating preterm labour
- PMID: 24809331
- PMCID: PMC7138067
- DOI: 10.1002/14651858.CD002860.pub2
Nitric oxide donors for treating preterm labour
Abstract
Background: A number of tocolytics have been advocated for the treatment of threatened preterm labour in order to delay birth. The rationale is that a delay in birth may be associated with improved neonatal morbidity or mortality. Nitric oxide donors, such as nitroglycerin, have been used to relax the uterus. This review addresses their efficacy, adverse effects and influence on neonatal outcome.
Objectives: To determine whether nitric oxide donors administered in threatened preterm labour are associated with a delay in birth, adverse effects or improved neonatal outcome.
Search methods: We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (1 December 2013).
Selection criteria: Randomised controlled trials of nitric oxide donors administered for tocolysis.
Data collection and analysis: Two review authors independently assessed trial quality and extracted data.
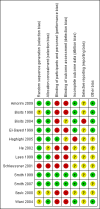
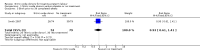
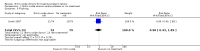
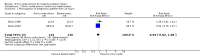
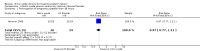
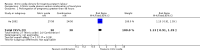
Main results: Twelve trials, including a total of 1227 women at risk of preterm labour, contributed data to this updated review. The methodological quality of trials was mixed; trials comparing nitric oxide donors with other types of tocolytics were not blinded and this may have had an impact on findings.Three studies compared nitric oxide donors (glyceryl trinitrate (GTN)) with placebo. There was no significant evidence that nitric oxide donors prolonged pregnancy beyond 48 hours (average risk ratio (RR) 1.19, 95% confidence interval (CI) 0.74 to 1.90, two studies, 186 women), and although for most adverse effects there was no significant difference between groups, women in the active treatment group in one study were at higher risk of experiencing a headache. For infant outcomes there was no significant evidence that nitric oxide donors reduced the risk of neonatal death or serious morbidity (stillbirth RR 0.36, 95% CI 0.01 to 8.59, one study, 153 infants; neonatal death RR 0.43, 95% CI 0.06 to 2.89, two studies, 186 infants). One study, using a composite outcome, reported a reduced risk of serious adverse outcomes for infants in the GTN group which approached statistical significance (RR 0.29, 95% CI 0.08 to 1.00, 153 infants). Overall, these studies were underpowered to identify differences between groups for most outcomes.When nitric oxide donors were compared with other tocolytic drugs there was no significant evidence that nitric oxide donors performed better than other tocolytics (betamimetics, magnesium sulphate, a calcium channel blocker or a combination of tocolytics) in terms of pregnancy prolongation, although nitric oxide donors appeared to be associated with a reduction in most adverse effects, apart from headache. There was no significant difference between groups for infant morbidity or mortality outcomes.
Authors' conclusions: There is currently insufficient evidence to support the routine administration of nitric oxide donors in the treatment of threatened preterm labour.
Conflict of interest statement
ST provides commercial consultancy advice. None of these companies make any of the drugs included in the review. KD has received honoraria from Merck, Bayer and Actavis. None of these companies produce tocolytics. TD has been paid from a grant from WHO for work on this review; WHO has had no influence over the findings or conclusions of the review.
Figures






















































Update of
-
Nitric oxide donors for the treatment of preterm labour.Cochrane Database Syst Rev. 2002;(3):CD002860. doi: 10.1002/14651858.CD002860. Cochrane Database Syst Rev. 2002. Update in: Cochrane Database Syst Rev. 2014 May 08;(5):CD002860. doi: 10.1002/14651858.CD002860.pub2. PMID: 12137661 Updated.
References
References to studies included in this review
Amorim 2009 {published data only}
-
- Amorim MM, Lippo LA, Costa AA, Coutinho IC, Souza AS. Transdermal nitroglycerin versus oral nifedipine administration for tocolysis: a randomized clinical trial [Nitroglicerina transdermica versus nifedipina oral para inibicao do trabalho de parto prematuro: ensaio clinico randomizado]. Revista Brasileira de Ginecologia e Obstetricia 2009;31(11):552‐8. - PubMed
Bisits 1998 {published data only}
-
- Bisits A, Madsen G, McLean M, O'Callaghan S, Smith R, Giles W. Corticotrophin‐releasing hormone: a biochemical predictor of preterm delivery in a pilot randomized trial of the treatment of preterm labor. American Journal of Obstetrics and Gynecology 1998;178:862‐6. - PubMed
Bisits 2004 {published data only}
-
- Bisits A, Madsen G, Knox M, Gill A, Smith R, Yeo G, et al. The Randomized Nitric Oxide Tocolysis Trial (RNOTT) for the treatment of preterm labor. American Journal of Obstetrics and Gynecology 2004;191(3):683‐90. - PubMed
-
- Giles W, Knox M, Madsden G, Bisits A, Gill A, Smith R, et al. The randomised nitric oxide tocolysis trial. American Journal of Obstetrics and Gynecology 2001;184(1):S6. - PubMed
-
- Gill A, Giles W, Bisits A, Madsen G, Knox M, Tudehope D, et al. Neonatal neurodevelopmental outcomes following tocolysis with glycerol trinitrate patches and iv beta 2 agonist therapy [abstract]. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S117. - PubMed
-
- Gill A, Madsen G, Knox M, Bisits A, Giles W, Tudehope D, et al. Neonatal neurodevelopmental outcomes following tocolysis with glycerol trinitrate patches. American Journal of Obstetrics and Gynecology 2006;195(2):484‐7. - PubMed
El‐Sayed 1999 {published data only}
-
- El‐Sayed YY, Riley ET, Holbrook RH, Cohen SE, Chitkara U, Druzin ML. Randomized comparison of intravenous nitroglycerin and magnesium sulfate for treatment of preterm labour. Obstetrics and Gynecology 1999;93:79‐83. - PubMed
Haghighi 2005 {published data only}
-
- Haghighi L, Akbarian A. Isosorbide dinitrate for treatment of preterm labor. International Journal of Gynecology & Obstetrics 2005;89:274‐5. - PubMed
He 2002 {published data only}
-
- He Q, Sha J, Gu Q, Gu H, Chen X, Yang Z, et al. Clinical effect and mechanism of nitroglycerin patch on arresting preterm labor. [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2002;37(3):134‐5. - PubMed
Lees 1999 {published data only}
-
- Black RS, Lees C, Thompson C, Pickles A, Campbell S. Maternal and fetal cardiovascular effects of transdermal glyceryl trinitrate and intravenous ritodrine. Obstetrics and Gynecology 1999;94(4):572‐6. - PubMed
-
- Lees CC, Lojacono A, Thompson C, Danti L, Black RS, Tanzi P, et al for the GTN preterm labour investigation group. Glyceryl trinitrate and ritodrine in tocolysis: an international multicenter randomized study. Obstetrics and Gynecology 1999;94(3):403‐8. - PubMed
Schleussner 2001 {published data only}
-
- Schleussner E, Moller A, Gross W, Kahler C, Moller U, Richter S, et al. Maternal and fetal side effects of tocolysis using transdermal nitroglycerin or intravenous fenoterol combined with magnesium sulfate. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;106:14‐9. - PubMed
-
- Schleussner E, Richter S, Gross W, Kahler C, Moller A, Moller U, et al. Glyceryl trinitrate patches versus fenoterol intravenous in tocolysis ‐ a prospective randomized trial [Nitroglyzerinpflaster zur Tocolyse ‐ ein prospectiv randomisierter Vergleich mit fenoterol per infusionem]. Zeitschrift fur Geburtshilfe und Neonatologie 2001;205:189‐94. - PubMed
Smith 1999 {published data only}
-
- Smith GN, Walker MC, McGrath MJ. Randomized double‐blind, placebo controlled trial assessing nitroglycerin as a tocolytic. American Journal of Obstetrics and Gynecology 1998;178(1 Pt 2):S181.
-
- Smith GN, Walker MC, McGrath MJ. Randomized double‐blind, placebo controlled trial assessing nitroglycerin as a tocolytic. British Journal of Obstetrics and Gynaecology 1999;106(7):736‐9. - PubMed
Smith 2007 {published data only}
-
- Guo Y, Longo CJ, Xie R, Wen SW, Walker MC, Smith GN. Cost‐effectiveness of transdermal nitroglycerin use for preterm labor. Value in Health 2011;14(2):240‐6. - PubMed
-
- Guo Y, Xie R, Wen SW, Walker MC, Smith GN. Maternal transdermal nitroglycerin use and early childhood development. Journal of Obstetrics and Gynaecology Canada 2010;32(12):1147‐52. - PubMed
-
- Smith G. Canadian preterm labour nitroglycerin trial. Personal communication 2002.
-
- Smith G. The Canadian preterm labour nitroglycerin trial. Current Controlled Trials (www.controlled‐trials.com/mrct) (accessed 2005).
-
- Smith G, Walker M, Ohlsson A, O'Brien K, Rory W. Transdermal nitroglycerin for preterm labor [abstract]. Pediatric Academic Societies Annual Meeting; 2006 April 29‐May 2; San Francisco, CA, USA. San Francisco, 2006.
Szulc 2000 {published data only}
-
- Szulc E, Leibschang J. Comparative evaluation of efficiency and tolerance of two alternative methods for premature uterine contractions suppression using fenoterol and nitroglycerin [Polish] [Ocena porownawcza skutecznoSci i tolerancji dwoch alternatywnych metod hamowania przedwczesnej czynnoSci skurczowej macicy za pomocy fenoterolu i nitrogliceryny.]. Medycyna Wieku Rozwojowego 2000;4(3):307‐16. - PubMed
Wani 2004 {published data only}
-
- Rath S. Use of glyceryl trinitrate for preterm labour in a maternity hospital in United Arab Emirates [abstract]. 49th All India Congress of Obstetrics and Gynaecology; 2006 January 6‐9; Cochin, Kerala State, India. 2006:49.
-
- Wani M, John S, Graham I. A comparison of glyceryl trinitrate and ritodrine for the treatment of threatened preterm labour. Women's Health ‐ into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town South Africa. London, UK: RCOG Press, 1999:62.
-
- Wani MP, Barakzai N, Graham I. Glyceryl trinitrate vs ritodrine for the treatment of preterm labor. International Journal of Gynecology & Obstetrics 2004;85:165‐7. - PubMed
References to studies excluded from this review
Bulgay‐Moerschel 2008 {published data only}
-
- Bulgay‐Moerschel M, Schneider U, Schleussner E. Tocolysis with nitroglycerin patches vs. fenoterol i.v. ‐ results of a randomized multicenter study. Journal of Maternal‐Fetal and Neonatal Medicine 2008;21(Suppl 1):115.
Clavin 1996 {published data only}
-
- Clavin DK, Bayhi DA, Nolan TE, Rigby FB, Cork RC, Miller JM. Comparison of intravenous magnesium sulfate and nitroglycerin for preterm labor: preliminary data [abstract]. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):307.
Groom 2000 {published data only}
-
- Groom KM, Bennett PR, Shennan AH. Randomised, double‐blind, placebo controlled pilot study assessing nitroglycerin as a tocolytic [letter]. BJOG: an international journal of obstetrics and gynaecology 2000;107(9):1182‐3. - PubMed
Hogberg 1998 {published data only}
-
- Hogberg U. Nitroglycerin and terbutalin versus placebo and terbutalin ‐ a randomized controlled study for preterm labour. Personal communication January 21 1998.
Leszczynska 2001 {published data only}
-
- Leszczynska‐Gorzelak B, Laskowska M, Marciniak B, Oleszczuk J. Nitric oxide for treatment of threatened preterm labour. International Journal of Gynecology and Obstetrics 2001;73(3):201‐6. - PubMed
Pasargiklian 1983 {published data only}
-
- Pasargiklian R, Monti G, Bertulessi C. Clenbuterol in the treatment of premature labor [Italian] [Il clenbuterolo nel trattamento del parto pretermine]. Minerva Ginecologica 1983;35:423‐9. - PubMed
Rytlewski 2008 {published data only}
-
- Rytlewski K, Olszanecki R, Lauterbach R, Grzyb A, Kiec‐Wilk B, Dembinska‐Kiec A, et al. Effects of oral L‐arginine on the pulsatility indices of umbilical artery and middle cerebral artery in preterm labor. European Journal of Obstetrics & Gynecology and Reproductive Biology 2008;138(1):23‐8. - PubMed
References to studies awaiting assessment
Simsek 2000 {published data only}
-
- Simsek T, Ozgur K, Sarlak O, Trak B, Uner M. Gliseryl trinitrate patches are as effective as ritodrin HCL in the treatment of preterm labor. Jinekoloji Ve Obstetri Bulteni 2000;9:63‐5.
Additional references
Anotayanonth 2004
Arthur 2008
Buhimschi 1995
-
- Buhimschi I, Yallampalli C, Dong YL, Garfield RE. Involvement of a nitric oxide‐cyclic guanosine‐monophosphate pathway in control of human uterine contractility in pregnancy. American Journal of Obstetrics and Gynecology 1995;172:1577‐84. - PubMed
Clarke 2000
-
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.1 [updated June 2000]. In: Review Manager (RevMan) [Computer program]. Version 4.1. Oxford, England: The Cochrane Collaboration, 2000.
Haas 2012
Higgins 2011
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
King 2002
King 2005
Kinney 2012
-
- Kinney MV, Howson CP, McDougall L, Lawn JE (editors). March of Dimes, PMNCH, Save the Children, WHO. Born Too Soon: The Global Action Report on Preterm Birth. Geneva: World Health Organization, 2012.
Momohara 2004
-
- Momohara Y, Sakamoto S, Obayashi S, Aso T, Goto M, Azuma H. Roles of endogenous nitric oxide synthase inhibitors and endothelin‐1 for regulating myometrial contractions during gestation in the rat. Molecular Human Reproduction 2004;7:505‐12. - PubMed
Norman 1996
-
- Norman J. Nitric oxide and the myometrium. Pharmacology and Therapeutics 1996;70:91‐100. - PubMed
Okawa 2005
-
- Okawa T, Asano K, Takahashi H, Sato A, Vedernikov YP, Saade GR, et al. Nitric oxide donor‐induced inhibition of pregnant rat uterine spontaneous contractile activity and release of nitric oxide from uterus measured by microdialysis. Journal of Endocrinological Investigation 2005;11:998‐1002. - PubMed
RCOG 2011
-
- Guidelines Committee of the Royal College of Obstetricians and Gynaecologists. Tocolysis for Women in Preterm Labour. Green‐top Guidelines 1B. London: RCOG, 2011.
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Roberts 2006
Sladek 1993
-
- Sladek SM, Regenstein AC, Lykins D, Roberts JM. Nitric oxide synthase activity in pregnant rabbit uterus decreases on the last day of pregnancy. American Journal of Obstetrics and Gynecology 1993;169:1285‐91. - PubMed
Stevenson 1996
Yallampalli 1993
-
- Yallampalli C, Garfield RE, Byam‐Smith M. Nitric oxide inhibits uterine contractility during pregnancy but not during delivery. Endocrinology 1993;133:1899‐902. - PubMed
Yallampalli 1994
-
- Yallampalli C, Izumi H, Byam‐Smith M, Garfield RE. An l‐arginine‐nitric oxide‐cyclic guanosine monophosphate system exists in the uterus and inhibits contractility during pregnancy. American Journal of Obstetrics and Gynecology 1994;170:175‐85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

