Fabrication of an electrochemical sensor based on gold nanoparticles/carbon nanotubes as nanocomposite materials: determination of myricetin in some drinks
- PMID: 24809346
- PMCID: PMC4014532
- DOI: 10.1371/journal.pone.0096686
Fabrication of an electrochemical sensor based on gold nanoparticles/carbon nanotubes as nanocomposite materials: determination of myricetin in some drinks
Abstract
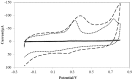
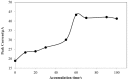
In this paper, the electrochemical behavior of myricetin on a gold nanoparticle/ethylenediamine/multi-walled carbon-nanotube modified glassy carbon electrode (AuNPs/en/MWCNTs/GCE) has been investigated. Myricetin effectively accumulated on the AuNPs/en/MWCNTs/GCE and caused a pair of irreversible redox peaks at around 0.408 V and 0.191 V (vs. Ag/AgCl) in 0.1 mol L-1 phosphate buffer solution (pH 3.5) for oxidation and reduction reactions respectively. The heights of the redox peaks were significantly higher on AuNPs/en/MWNTs/GCE compare with MWCNTs/GC and there was no peak on bare GC. The electron-transfer reaction for myricetin on the surface of electrochemical sensor was controlled by adsorption. Some parameters including pH, accumulation potential, accumulation time and scan rate have been optimized. Under the optimum conditions, anodic peak current was proportional to myricetin concentration in the dynamic range of 5.0×10-8 to 4.0×10-5 mol L-1 with the detection limit of 1.2×10-8 mol L-1. The proposed method was successfully used for the determination of myricetin content in tea and fruit juices.
Conflict of interest statement
Figures


Similar articles
-
Fabrication of an electrochemical sensor based on carbon nanotubes modified with gold nanoparticles for determination of valrubicin as a chemotherapy drug: valrubicin-DNA interaction.Mater Sci Eng C Mater Biol Appl. 2015 Apr;49:769-775. doi: 10.1016/j.msec.2015.01.072. Epub 2015 Jan 24. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 25687007
-
An electrochemical sensor for the determination of tartrazine based on CHIT/GO/MWCNTs/AuNPs composite film modified glassy carbon electrode.Drug Chem Toxicol. 2021 Sep;44(5):447-457. doi: 10.1080/01480545.2019.1601210. Epub 2019 Apr 25. Drug Chem Toxicol. 2021. PMID: 31020858
-
A disposable electrochemical immunosensor for carcinoembryonic antigen based on nano-Au/multi-walled carbon nanotubes-chitosans nanocomposite film modified glassy carbon electrode.Anal Chim Acta. 2010 Feb 5;659(1-2):102-8. doi: 10.1016/j.aca.2009.11.023. Epub 2009 Nov 17. Anal Chim Acta. 2010. PMID: 20103110
-
A sensitive nanocomposite design via carbon nanotube and silver nanoparticles: Selective probing of Emedastine Difumarate.J Pharm Biomed Anal. 2020 Mar 20;181:113096. doi: 10.1016/j.jpba.2020.113096. Epub 2020 Jan 3. J Pharm Biomed Anal. 2020. PMID: 32014685
-
Simple flow injection for determination of sulfite by amperometric detection using glassy carbon electrode modified with carbon nanotubes-PDDA-gold nanoparticles.Talanta. 2015 Feb;133:134-41. doi: 10.1016/j.talanta.2014.07.055. Epub 2014 Jul 31. Talanta. 2015. PMID: 25435239
Cited by
-
Construction of an Electrochemical Sensor Based on Carbon Nanotubes/Gold Nanoparticles for Trace Determination of Amoxicillin in Bovine Milk.Sensors (Basel). 2016 Jan 20;16(1):56. doi: 10.3390/s16010056. Sensors (Basel). 2016. PMID: 26805829 Free PMC article.
-
Gold Nanomaterials-Based Electrochemical Sensors and Biosensors for Phenolic Antioxidants Detection: Recent Advances.Nanomaterials (Basel). 2022 Mar 14;12(6):959. doi: 10.3390/nano12060959. Nanomaterials (Basel). 2022. PMID: 35335772 Free PMC article. Review.
-
Electrochemical synthesis of a nanocomposite consisting of carboxy-modified multi-walled carbon nanotubes, polythionine and platinum nanoparticles for simultaneous voltammetric determination of myricetin and rutin.Mikrochim Acta. 2018 Aug 16;185(9):414. doi: 10.1007/s00604-018-2947-7. Mikrochim Acta. 2018. PMID: 30116901
-
Role of Au(NPs) in the enhanced response of Au(NPs)-decorated MWCNT electrochemical biosensor.Int J Nanomedicine. 2018 Apr 17;13:2093-2106. doi: 10.2147/IJN.S155388. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29713161 Free PMC article.
-
A screen printed carbon electrode modified with carbon nanotubes and gold nanoparticles as a sensitive electrochemical sensor for determination of thiamphenicol residue in milk.RSC Adv. 2018 Jan 11;8(5):2714-2722. doi: 10.1039/c7ra07544h. eCollection 2018 Jan 9. RSC Adv. 2018. PMID: 35541441 Free PMC article.
References
-
- Chen S, Murray RW (1999) Electrochemical quantized capacitance charging of surface ensembles of gold nanoparticles. J Phys Chem B 103: 9996–10000.
-
- Li J, Yamada Y, Murakoshi K, Nakato Y (2001) Sustainable metal nano-contacts showing quantized conductance prepared at a gap of thin metal wires in solution. Chem Commun 21: 2170–2171. - PubMed
-
- Haruta M (1997) Size- and support-dependency in the catalysis of gold. Catal Today 36: 153–166.
-
- Subramanian V, Wolf EE, Kamat PV (2003) Green emission to probe photoinduced charging events in ZnO−Au nanoparticles. Charge distribution and fermi-level equilibration. J Phys Chem B 107: 7479–7485.
-
- Welch CW, Compton RG (2006) The use of nanoparticles in electroanalysis: a review. Anal Bioanal Chem 384: 601–619. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

