Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes
- PMID: 24809394
- PMCID: PMC4082168
- DOI: 10.2174/1573399810666140508121012
Leptin- and leptin receptor-deficient rodent models: relevance for human type 2 diabetes
Abstract
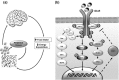
Among the most widely used animal models in obesity-induced type 2 diabetes mellitus (T2DM) research are the congenital leptin- and leptin receptor-deficient rodent models. These include the leptin-deficient ob/ob mice and the leptin receptor-deficient db/db mice, Zucker fatty rats, Zucker diabetic fatty rats, SHR/N-cp rats, and JCR:LA-cp rats. After decades of mechanistic and therapeutic research schemes with these animal models, many species differences have been uncovered, but researchers continue to overlook these differences, leading to untranslatable research. The purpose of this review is to analyze and comprehensively recapitulate the most common leptin/leptin receptor-based animal models with respect to their relevance and translatability to human T2DM. Our analysis revealed that, although these rodents develop obesity due to hyperphagia caused by abnormal leptin/leptin receptor signaling with the subsequent appearance of T2DM-like manifestations, these are in fact secondary to genetic mutations that do not reflect disease etiology in humans, for whom leptin or leptin receptor deficiency is not an important contributor to T2DM. A detailed comparison of the roles of genetic susceptibility, obesity, hyperglycemia, hyperinsulinemia, insulin resistance, and diabetic complications as well as leptin expression, signaling, and other factors that confound translation are presented here. There are substantial differences between these animal models and human T2DM that limit reliable, reproducible, and translatable insight into human T2DM. Therefore, it is imperative that researchers recognize and acknowledge the limitations of the leptin/leptin receptor- based rodent models and invest in research methods that would be directly and reliably applicable to humans in order to advance T2DM management.
Figures
References
-
- National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. http: //www.cdc.gov/DIABETES/pubs/factsheet11.htm. 2011.
-
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. - PubMed
-
- Screening for Type 2 Diabetes: report of a World Health Organization and International Diabetes Federation Meeting. http: //www.who.int/diabetes/publications/en/screening_mnc03.pdf. 2003.
-
- Lee AW, Cox RD. Use of mouse models in studying type 2 diabetes mellitus. Expert Rev Mol Med. 2011;13:e1. - PubMed
-
- Chatzigeorgiou A, Halapas A, Kalafatakis K, Kamper E. The use of animal models in the study of diabetes mellitus. In Vivo. 2009;23(2):245–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

