Synonymous constraint elements show a tendency to encode intrinsically disordered protein segments
- PMID: 24809503
- PMCID: PMC4014394
- DOI: 10.1371/journal.pcbi.1003607
Synonymous constraint elements show a tendency to encode intrinsically disordered protein segments
Abstract
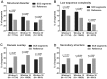
Synonymous constraint elements (SCEs) are protein-coding genomic regions with very low synonymous mutation rates believed to carry additional, overlapping functions. Thousands of such potentially multi-functional elements were recently discovered by analyzing the levels and patterns of evolutionary conservation in human coding exons. These elements provide a good opportunity to improve our understanding of how the redundant nature of the genetic code is exploited in the cell. Our premise is that the protein segments encoded by such elements might better comply with the increased functional demands if they are structurally less constrained (i.e. intrinsically disordered). To test this idea, we investigated the protein segments encoded by SCEs with computational tools to describe the underlying structural properties. In addition to SCEs, we examined the level of disorder, secondary structure, and sequence complexity of protein regions overlapping with experimentally validated splice regulatory sites. We show that multi-functional gene regions translate into protein segments that are significantly enriched in structural disorder and compositional bias, while they are depleted in secondary structure and domain annotations compared to reference segments of similar lengths. This tendency suggests that relaxed protein structural constraints provide an advantage when accommodating multiple overlapping functions in coding regions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Ureta-Vidal A, Ettwiller L, Birney E (2003) Comparative genomics: genome-wide analysis in metazoan eukaryotes. Nat Rev Genet 4: 251–262. - PubMed
-
- Sidow A (2002) Sequence first. Ask questions later. Cell 111: 13–16. - PubMed
-
- Sumiyama K, Kim CB, Ruddle FH (2001) An efficient cis-element discovery method using multiple sequence comparisons based on evolutionary relationships. Genomics 71: 260–262. - PubMed
-
- Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, et al. (2005) Phylogenetic shadowing and computational identification of human microRNA genes. Cell 120: 21–24. - PubMed
-
- Lim LP, Glasner ME, Yekta S, Burge CB, Bartel DP (2003) Vertebrate microRNA genes. Science 299: 1540. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

