Brain remodelling following endothelin-1 induced stroke in conscious rats
- PMID: 24809543
- PMCID: PMC4029108
- DOI: 10.1371/journal.pone.0097007
Brain remodelling following endothelin-1 induced stroke in conscious rats
Abstract
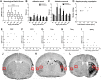
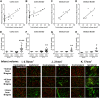
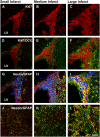
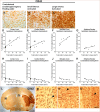
The extent of stroke damage in patients affects the range of subsequent pathophysiological responses that influence recovery. Here we investigate the effect of lesion size on development of new blood vessels as well as inflammation and scar formation and cellular responses within the subventricular zone (SVZ) following transient focal ischemia in rats (n = 34). Endothelin-1-induced stroke resulted in neurological deficits detected between 1 and 7 days (P<0.001), but significant recovery was observed beyond this time. MCID image analysis revealed varying degrees of damage in the ipsilateral cortex and striatum with infarct volumes ranging from 0.76-77 mm3 after 14 days, where larger infarct volumes correlated with greater functional deficits up to 7 days (r = 0.53, P<0.05). Point counting of blood vessels within consistent sample regions revealed that increased vessel numbers correlated significantly with larger infarct volumes 14 days post-stroke in the core cortical infarct (r = 0.81, P<0.0001), core striatal infarct (r = 0.91, P<0.005) and surrounding border zones (r = 0.66, P<0.005; and r = 0.73, P<0.05). Cell proliferation within the SVZ also increased with infarct size (P<0.01) with a greater number of Nestin/GFAP positive cells observed extending towards the border zone in rats with larger infarcts. Lesion size correlated with both increased microglia and astrocyte activation, with severely diffuse astrocyte transition, the formation of the glial scar being more pronounced in rats with larger infarcts. Thus stroke severity affects cell proliferation within the SVZ in response to injury, which may ultimately make a further contribution to glial scar formation, an important factor to consider when developing treatment strategies that promote neurogenesis.
Conflict of interest statement
Figures

References
-
- Dirnagl U, Iadecola C, Moskowitz MA (1999) Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci 22: 391–397. - PubMed
-
- Lo EH, Dalkara T, Moskowitz MA (2003) Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 4: 399–415. - PubMed
-
- Huang IJ, Chen CY, Chung HW, Chang DC, Lee CC, et al. (2001) Time course of cerebral infarction in the middle cerebral arterial territory: deep watershed versus territorial subtypes on diffusion-weighted MR images. Radiology 221: 35–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

