Periostin secreted by mesenchymal stem cells supports tendon formation in an ectopic mouse model
- PMID: 24809660
- PMCID: PMC4120815
- DOI: 10.1089/scd.2014.0124
Periostin secreted by mesenchymal stem cells supports tendon formation in an ectopic mouse model
Abstract
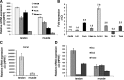
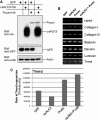
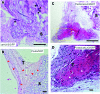
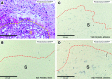
True tendon regeneration in human patients remains a vision of musculoskeletal therapies. In comparison to other mesenchymal lineages the biology of tenogenic differentiation is barely understood. Specifically, easy and efficient protocols are lacking that might enable tendon cell and tissue differentiation based on adult (stem) cell sources. In the murine mesenchymal progenitor cell line C3H10T½, overexpression of the growth factor bone morphogenetic protein 2 (BMP2) and a constitutively active transcription factor, Smad8 L+MH2, mediates tendon cell differentiation in vitro and the formation of tendon-like tissue in vivo. We hypothesized that during this differentiation secreted factors involved in extracellular matrix formation exert a major impact on tendon development. Gene expression analyses revealed four genes encoding secreted factors that are notably upregulated: periostin, C-type lectin domain family 3 (member b), RNase A4, and follistatin-like 1. These factors have not previously been implicated in tendon biology. Among these, periostin showed a specific expression in tenocytes of adult mouse Achilles tendon and in chondrocytes within the nonmineralized fibrocartilage zone of the enthesis with the calcaneus. Overexpression of periostin alone or in combination with constitutively active BMP receptor type in human mesenchymal stem cells and subsequent implantation into ectopic sites in mice demonstrated a reproducible moderate tenogenic capacity that has not been described before. Therefore, periostin may belong to the factors contributing to the development of tenogenic tissue.
Figures


Similar articles
-
Mesenchymal stem cell-dependent formation of heterotopic tendon-bone insertions (osteotendinous junctions).Stem Cells. 2010 Sep;28(9):1590-601. doi: 10.1002/stem.487. Stem Cells. 2010. PMID: 20882636
-
Neotendon formation induced by manipulation of the Smad8 signalling pathway in mesenchymal stem cells.J Clin Invest. 2006 Apr;116(4):940-52. doi: 10.1172/JCI22689. J Clin Invest. 2006. PMID: 16585960 Free PMC article.
-
Stepwise Differentiation of Mesenchymal Stem Cells Augments Tendon-Like Tissue Formation and Defect Repair In Vivo.Stem Cells Transl Med. 2016 Aug;5(8):1106-16. doi: 10.5966/sctm.2015-0215. Epub 2016 Jun 8. Stem Cells Transl Med. 2016. PMID: 27280798 Free PMC article.
-
Biomaterial Properties and Differentiation Strategies for Tenogenic Differentiation of Mesenchymal Stem Cells.Cells. 2025 Mar 18;14(6):452. doi: 10.3390/cells14060452. Cells. 2025. PMID: 40136701 Free PMC article. Review.
-
The role of periostin in tissue remodeling across health and disease.Cell Mol Life Sci. 2014 Apr;71(7):1279-88. doi: 10.1007/s00018-013-1494-y. Epub 2013 Oct 22. Cell Mol Life Sci. 2014. PMID: 24146092 Free PMC article. Review.
Cited by
-
Periostin as a multifunctional modulator of the wound healing response.Cell Tissue Res. 2016 Sep;365(3):453-65. doi: 10.1007/s00441-016-2426-6. Epub 2016 May 28. Cell Tissue Res. 2016. PMID: 27234502 Free PMC article. Review.
-
Mechanisms of tissue degeneration mediated by periostin in spinal degenerative diseases and their implications for pathology and diagnosis: a review.Front Med (Lausanne). 2023 Oct 31;10:1276900. doi: 10.3389/fmed.2023.1276900. eCollection 2023. Front Med (Lausanne). 2023. PMID: 38020106 Free PMC article. Review.
-
In vivo ligamentogenesis in embroidered poly(lactic-co-ε-caprolactone) / polylactic acid scaffolds functionalized by fluorination and hexamethylene diisocyanate cross-linked collagen foams.Histochem Cell Biol. 2023 Mar;159(3):275-292. doi: 10.1007/s00418-022-02156-3. Epub 2022 Oct 29. Histochem Cell Biol. 2023. PMID: 36309635 Free PMC article.
-
The "other" 15-40%: The Role of Non-Collagenous Extracellular Matrix Proteins and Minor Collagens in Tendon.J Orthop Res. 2020 Jan;38(1):23-35. doi: 10.1002/jor.24440. Epub 2019 Aug 26. J Orthop Res. 2020. PMID: 31410892 Free PMC article. Review.
-
Periostin: An Emerging Molecule With a Potential Role in Spinal Degenerative Diseases.Front Med (Lausanne). 2021 Aug 27;8:694800. doi: 10.3389/fmed.2021.694800. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34513869 Free PMC article. Review.
References
-
- Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF. and Soslowsky LJ. (2006). Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res 24:2124–2132 - PubMed
-
- Hoffmann A. and Gross G. (2009). Innovative strategies for treatment of soft tissue injuries in human and animal athletes. Med Sport Sci 54:150–165 - PubMed
-
- Chen X, Song XH, Yin Z, Zou XH, Wang LL, Hu H, Cao T, Zheng M. and Ouyang HW. (2009). Stepwise differentiation of human embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells 27:1276–1287 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

