Epstein-Barr virus down-regulates tumor suppressor DOK1 expression
- PMID: 24809689
- PMCID: PMC4014463
- DOI: 10.1371/journal.ppat.1004125
Epstein-Barr virus down-regulates tumor suppressor DOK1 expression
Abstract
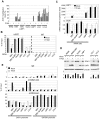
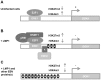
The DOK1 tumor suppressor gene encodes an adapter protein that acts as a negative regulator of several signaling pathways. We have previously reported that DOK1 expression is up-regulated upon cellular stress, via the transcription factor E2F1, and down-regulated in a variety of human malignancies due to aberrant hypermethylation of its promoter. Here we show that Epstein Barr virus (EBV) infection of primary human B-cells leads to the down-regulation of DOK1 gene expression via the viral oncoprotein LMP1. LMP1 alone induces recruitment to the DOK1 promoter of at least two independent inhibitory complexes, one containing E2F1/pRB/DNMT1 and another containing at least EZH2. These events result in tri-methylation of histone H3 at lysine 27 (H3K27me3) of the DOK1 promoter and gene expression silencing. We also present evidence that the presence of additional EBV proteins leads to further repression of DOK1 expression with an additional mechanism. Indeed, EBV infection of B-cells induces DNA methylation at the DOK1 promoter region including the E2F1 responsive elements that, in turn, lose the ability to interact with E2F complexes. Treatment of EBV-infected B-cell-lines with the methyl-transferase inhibitor 5-aza-2'-deoxycytidine rescues DOK1 expression. In summary, our data show the deregulation of DOK1 gene expression by EBV and provide novel insights into the regulation of the DOK1 tumor suppressor in viral-related carcinogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Mashima R, Hishida Y, Tezuka T, Yamanashi Y (2009) The roles of Dok family adapters in immunoreceptor signaling. Immunol Rev 232: 273–285. - PubMed
-
- Yasuda T, Bundo K, Hino A, Honda K, Inoue A, et al. (2007) Dok-1 and Dok-2 are negative regulators of T cell receptor signaling. Int Immunol 19: 487–495. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

