Anti-vascular endothelial growth factor therapy-induced glioma invasion is associated with accumulation of Tie2-expressing monocytes
- PMID: 24809734
- PMCID: PMC4039157
- DOI: 10.18632/oncotarget.1893
Anti-vascular endothelial growth factor therapy-induced glioma invasion is associated with accumulation of Tie2-expressing monocytes
Abstract
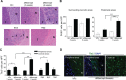
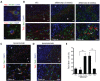
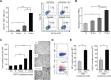
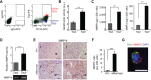
The addition of anti-angiogenic therapy to the few treatments available to patients with malignant gliomas was based on the fact that these tumors are highly vascularized and on encouraging results from preclinical and clinical studies. However, tumors that initially respond to this therapy invariably recur with the acquisition of a highly aggressive and invasive phenotype. Although several myeloid populations have been associated to this pattern of recurrence, a specific targetable population has not been yet identified. Here, we present evidence for the accumulation of Tie2-expressing monocytes/macrophages (TEMs) at the tumor/normal brain interface of mice treated with anti-VEGF therapies in regions with heightened tumoral invasion. Furthermore, we describe the presence of TEMs in malignant glioma surgical specimens that recurred after bevacizumab treatment. Our studies showed that TEMs enhanced the invasive properties of glioma cells and secreted high levels of gelatinase enzymatic proteins. Accordingly, Tie2⁺MMP9⁺ monocytic cells were consistently detected in the invasive tumor edge upon anti-VEGF therapies. Our results suggest the presence of a specific myeloid/monocytic subpopulation that plays a pivotal role in the mechanism of escape of malignant gliomas from anti-VEGF therapies and therefore constitutes a new cellular target for combination therapies in patients selected for anti-angiogenesis treatment.
Figures

References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, Garren N, Mackey M, Butman JA, Camphausen K, Park J, Albert PS, Fine HA. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–745. - PMC - PubMed
-
- Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, Yung WK, Paleologos N, Nicholas MK, Jensen R, Vredenburgh J, Huang J, Zheng M, Cloughesy T. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–4740. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

