Audio-visual presentation of information for informed consent for participation in clinical trials
- PMID: 24809816
- PMCID: PMC6599866
- DOI: 10.1002/14651858.CD003717.pub3
Audio-visual presentation of information for informed consent for participation in clinical trials
Abstract
Background: Informed consent is a critical component of clinical research. Different methods of presenting information to potential participants of clinical trials may improve the informed consent process. Audio-visual interventions (presented, for example, on the Internet or on DVD) are one such method. We updated a 2008 review of the effects of these interventions for informed consent for trial participation.
Objectives: To assess the effects of audio-visual information interventions regarding informed consent compared with standard information or placebo audio-visual interventions regarding informed consent for potential clinical trial participants, in terms of their understanding, satisfaction, willingness to participate, and anxiety or other psychological distress.
Search methods: We searched: the Cochrane Central Register of Controlled Trials (CENTRAL), The Cochrane Library, issue 6, 2012; MEDLINE (OvidSP) (1946 to 13 June 2012); EMBASE (OvidSP) (1947 to 12 June 2012); PsycINFO (OvidSP) (1806 to June week 1 2012); CINAHL (EbscoHOST) (1981 to 27 June 2012); Current Contents (OvidSP) (1993 Week 27 to 2012 Week 26); and ERIC (Proquest) (searched 27 June 2012). We also searched reference lists of included studies and relevant review articles, and contacted study authors and experts. There were no language restrictions.
Selection criteria: We included randomised and quasi-randomised controlled trials comparing audio-visual information alone, or in conjunction with standard forms of information provision (such as written or verbal information), with standard forms of information provision or placebo audio-visual information, in the informed consent process for clinical trials. Trials involved individuals or their guardians asked to consider participating in a real or hypothetical clinical study. (In the earlier version of this review we only included studies evaluating informed consent interventions for real studies).
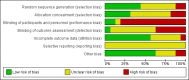
Data collection and analysis: Two authors independently assessed studies for inclusion and extracted data. We synthesised the findings using meta-analysis, where possible, and narrative synthesis of results. We assessed the risk of bias of individual studies and considered the impact of the quality of the overall evidence on the strength of the results.
Main results: We included 16 studies involving data from 1884 participants. Nine studies included participants considering real clinical trials, and eight included participants considering hypothetical clinical trials, with one including both. All studies were conducted in high-income countries.There is still much uncertainty about the effect of audio-visual informed consent interventions on a range of patient outcomes. However, when considered across comparisons, we found low to very low quality evidence that such interventions may slightly improve knowledge or understanding of the parent trial, but may make little or no difference to rate of participation or willingness to participate. Audio-visual presentation of informed consent may improve participant satisfaction with the consent information provided. However its effect on satisfaction with other aspects of the process is not clear. There is insufficient evidence to draw conclusions about anxiety arising from audio-visual informed consent. We found conflicting, very low quality evidence about whether audio-visual interventions took more or less time to administer. No study measured researcher satisfaction with the informed consent process, nor ease of use.The evidence from real clinical trials was rated as low quality for most outcomes, and for hypothetical studies, very low. We note, however, that this was in large part due to poor study reporting, the hypothetical nature of some studies and low participant numbers, rather than inconsistent results between studies or confirmed poor trial quality. We do not believe that any studies were funded by organisations with a vested interest in the results.
Authors' conclusions: The value of audio-visual interventions as a tool for helping to enhance the informed consent process for people considering participating in clinical trials remains largely unclear, although trends are emerging with regard to improvements in knowledge and satisfaction. Many relevant outcomes have not been evaluated in randomised trials. Triallists should continue to explore innovative methods of providing information to potential trial participants during the informed consent process, mindful of the range of outcomes that the intervention should be designed to achieve, and balancing the resource implications of intervention development and delivery against the purported benefits of any intervention.More trials, adhering to CONSORT standards, and conducted in settings and populations underserved in this review, i.e. low- and middle-income countries and people with low literacy, would strengthen the results of this review and broaden its applicability. Assessing process measures, such as time taken to administer the intervention and researcher satisfaction, would inform the implementation of audio-visual consent materials.
Conflict of interest statement
Authors included staff of the Cochrane Consumers and Communication Review Group (AS, MP, RR), based at La Trobe University and funded by the National Health and Medical Research Council (AS, RR) and the Department of Health (Victoria) (MP). The decision whether or not to publish the updated review did not involve these staff.
Figures












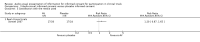
Update of
-
Audio-visual presentation of information for informed consent for participation in clinical trials.Cochrane Database Syst Rev. 2008 Jan 23;(1):CD003717. doi: 10.1002/14651858.CD003717.pub2. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2014 May 09;(5):CD003717. doi: 10.1002/14651858.CD003717.pub3. PMID: 18254029 Updated.
References
References to studies included in this review
Agre 2003a {published and unpublished data}
-
- Agre P, Rapkin B. Improving informed consent: a comparison of four consent tools. IRB: Ethics and Human Research 2003;25(6):1‐7. - PubMed
Benson 1988 {published and unpublished data}
-
- Benson PR, Appelbaum PS, Lidz CW, Winslade WJ. Information disclosure, subject understanding, and informed consent in psychiatric research. Law and Human Behavior 1988;12:455‐75. - PubMed
Campbell 2004 {published and unpublished data}
-
- Campbell FA, Goldman BD, Boccia ML, Skinner M. The effect of format modifications and reading comprehension on recall of informed consent information by low‐income parents: a comparison of print, video, and computer‐based presentations. Patient Education and Counseling 2004;53:205‐16. - PubMed
Harmell 2012 {published and unpublished data}
Hoffner 2012 {published data only (unpublished sought but not used)}
-
- Hitchcock‐Bryan S, Hoffner B, Joffe S, Powell M, Parker C, Wolanski A, et al. Entering a Clinical Trial: Is It Right For You?‐ A randomized study of the clinical trials video and its impact on the informed consent process [Abstract No. 9072]. Journal of Clinical Oncology : ASCO annual meeting proceedings. 2007; Vol. 18S Part I:510.
Hutchison 2007 {published and unpublished data}
-
- Hutchison C, Cowan C, Paul J. Patient understanding of research: developing and testing of a new questionnaire. European Journal of Cancer Care 2007;16:187‐96. - PubMed
-
- Hutchison C, MacCreaddie M. The process of developing audiovisual patient information: challenges and opportunities. Journal of Clinical Nursing 2007;16:2047‐55. - PubMed
Jeste 2009 {published data only}
Karunaratne 2010 {published and unpublished data}
-
- Karunaratne AS, Korenman SG, Thomas SL, Myles PS, Komesaroff PA. Improving communication when seeking informed consent: a randomised controlled study of a computer‐based method for providing information to prospective clinical trial participants. Medical Journal of Australia 2010;192:388‐92. - PubMed
Kass 2009 {published data only (unpublished sought but not used)}
-
- Daugherty C, Kass N, Fogarty LA, Sugarman J, Hlubocky F. A randomised trial of a CD‐ROM educational intervention for advanced cancer patients enrolling in early phase trials. Proceedings of the American Society for Clinical Oncology 2003;22:521.
-
- Hlubocky F, Kass N, Fogarty L. Effects of a CD‐ROM educational intervention (CD) on prognosis (prog) understanding among advanced cancer patients (acp) enrolling in early phase clinical trials. Journal of Clinical Oncology, 2004 ASCO Annual Meeting Proceedings 2004;22(14(S)):6054.
Klein 2012 {published data only (unpublished sought but not used)}
-
- Klein DW, Schartz HA. Instructional Strategies to Improve Informed Consent in Healthcare Research: Pilot Study of Interactivity and Multimedia. Paper presented at the American Educational Research Association National Conference Vancouver, Canada. April 2012.
Mittal 2007 {published and unpublished data}
-
- Mittal D, Palmer B, Dunn L, Landes R, Ghormley C, Beck C, et al. Comparison of two enhanced consent procedures for patients with mild Alzheimer disease or mild cognitive impairment. American Journal of Geriatric Psychiatry 2007;15(2):163‐7. - PubMed
Norris 1990 {published data only}
-
- Norris DR, Phillips MR. Using instructive videotapes to increase patient comprehension of informed consent. Journal of Clinical Research & Pharmacoepidemiology 1990;4(4):263‐8.
O'Lonergan 2011 {published data only (unpublished sought but not used)}
Strevel 2007 {published and unpublished data}
-
- Strevel EL, Newman C, Pond GR, MacLean M, Siu LL. The impact of an educational DVD on cancer patients considering participation in a phase I clinical trial. Supportive Care in Cancer 2007;15:829‐40. - PubMed
Weston 1997 {published and unpublished data}
-
- Weston J, Downes J, Hannah M. Evaluating the benefits of patient video during the informed consent process. Controlled Clinical Trials. 1995; Vol. 16:73S (abstract).
-
- Weston J, Hannah M, Downes J. Evaluating the benefits of a patient information video during the informed consent process. Patient Education & Counseling 1997;30(3):239‐45. - PubMed
Wirshing 2005 {published and unpublished data}
-
- Mintz J, Wirshing DA, et al. cited in Agre 2003.
-
- Wirshing DA, Mintz J, Kern R, Boyd J. A videotape intervention to enhance the informed consent process for patients participating in clinical research trial. Schizophrenia Research 2003;60:306.
-
- Wirshing DA, Sergi MJ, Mintz J. A videotape intervention to enhance the informed consent process for medical and psychiatric treatment research. American Journal of Psychiatry 2005;162(1):186‐8. - PubMed
References to studies excluded from this review
Barbour 1978 {published data only}
-
- Barbour GL, Blumenkrantz MJ. Videotape aids informed consent decision. JAMA 1978;240(25):2741‐2. - PubMed
Benitez 2002 {published data only}
-
- Benitez O, Devaux D, Dausset J. Audiovisual documentation of oral consent: a new method of informed consent for illiterate populations. Lancet 2002;359(9315):1406‐7. - PubMed
Benson 1985 {published data only}
-
- Benson PR, Roth LH, Winslade WJ. Informed consent in psychiatric research: preliminary findings from an ongoing investigation. Social Science & Medicine. 1985;20(12):1331‐41. - PubMed
Bickmore 2009 {published data only}
-
- National Institute of General Medical Sciences. Informed consent template for genetic repository research. available at: http://ccr.coriell.org/Sections/Support/NIGMS/Model.aspx?PgId=216 n.d. [2009].
Campbell 2008 {published data only}
-
- Campbell HM, Raisch DW, Sather MR, Segal AR, Warren AR, Naik R. Impact of a clinical trials information handbook on patient knowledge, perceptions, and likelihood of participation. IRB: Ethics & Human Research 2008;30:6‐14. - PubMed
Curbow 2004 {published data only}
-
- Curbow B, Fogarty LA, McDonnell K, Chill J, Scott LB. Can a brief video intervention improve breast cancer clinical trial knowledge and beliefs?. Social Science & Medicine 2004;58(1):193‐205. - PubMed
Dobscha 2005 {published data only}
-
- Dobscha SK, Corson K, Solodky J, Gerrity MS. Use of videoconferencing for depression research: enrollment, retention, and patient satisfaction. Telemedicine Journal & E Health 2005;11:84‐9. - PubMed
Du 2008 {published data only}
-
- Du W, Mood D, Gadgeel S, Simon MS. An educational video to increase clinical trials enrollment among lung cancer patients. Journal of Thoracic Oncology 2008;3:23‐9. - PubMed
Dunbar 1989 {published data only}
-
- Dunbar J, Cleary PA, Siebert C, Baker L, Brink S, Nathan DM. Implementation of a multicomponent process to obtain informed consent in the Diabetes Control and Complications Trial. Controlled Clinical Trials 1989;10(1):83‐96. - PubMed
Dunlop 2011 {published data only}
Dunn 2001 {published and unpublished data}
-
- Dunn LB, Lindamer LA, Palmer BW, Golshan S, Schneiderman LJ, Jeste DV. Improving understanding of research consent in middle‐aged and elderly patients with psychotic disorders. American Journal of Geriatric Psychiatry 2002;10(2):142‐50. - PubMed
-
- Dunn LB, Lindamer LA, Palmer BW, Schneidermann LJ, Jeste DV. Enhancing comprehension of consent for research in older patients with psychosis: a randomised study of a novel consent procedure. American Journal of Psychiatry 2001;158(11):1911‐3. - PubMed
Flory 2004 {published data only}
-
- Flory J, Emanuel E. Interventions to improve research participants' understanding in informed consent for research: a systematic review. JAMA 2004;292(13):1593‐601. - PubMed
Fureman 1997 {published data only}
-
- Fureman I, Meyers K, McLellan AT, Metzger D, Woody G. Evaluation of a video‐supplement to informed consent: injection drug users and preventive HIV vaccine efficacy trials. AIDS Education & Prevention 1997;9(4):330‐41. - PubMed
Gesualdo 2012 {published data only}
Goldberger 2011 {published data only}
-
- Goldberger JJ, Kruse J, Kadish AH, Passman R, Bergner DW. Effect of informed consent format on patient anxiety, knowledge, and satisfaction. American Heart Journal 2011;162:780‐5. - PubMed
Hack 2007 {published data only}
-
- Hack TF, Whelan T, Olivotto IA, Weir L, Bultz BD, Magwood B, et al. Standardized audiotape versus recorded consultation to enhance informed consent to a clinical trial in breast oncology. Psycho‐Oncology 2007;16:371‐6. - PubMed
Harzstark 2001 {published data only}
-
- Harzstark AL, Schurman CR, Luce J, Carlson RW. Studying the effectiveness of videos in conveying medical information regarding breast cancer risks and the Study of Tamoxifen and Raloxifene (STAR) trial. Breast Cancer Research & Treatment 2001;69:268.
Hazen 2010 {published data only}
-
- Hazen RA, Eder M, Drotar D, Zyzanski S, Reynolds AE, Reynolds CP, et al. Multi‐Site Intervention Study to Improve Consent Research Team. A feasibility trial of a video intervention to improve informed consent for parents of children with leukemia. Pediatric Blood & Cancer 2010;55:113‐8. - PMC - PubMed
Hendrikson 2007 {published data only}
-
- Hendrickson SG. Video recruitment of non‐English‐speaking participants. Western Journal of Nursing Research 2007;29:232‐42. - PubMed
Henry 2009 {published data only}
Hougham 2003a {published data only}
-
- Hougham GW, Sachs GA, Danner D, Mintz J, Patterson M, Roberts LW, et al. Empirical research on informed consent with the cognitively impaired. IRB: Human Ethics and Research (Supplement) 2003;25:1‐7. - PubMed
Hultgren 2009 {published data only}
-
- Hultgren B, Zaghi S, Carvas M, Nascimento B, Kwiatkowski J, Fregni F. Challenges in consenting subjects for studies with brain stimulation: feasibility of multimedia video use during the informed consent process. Brain Stimulation 2009;2:174–8. - PubMed
Ishii 2007 {published data only}
-
- Ishii M, Ohashi Y. Influence of an educational videotape on attitudes toward participating in cohort studies‐‐results of a randomized controlled trial. Japanese journal of public health 2007;54:419‐26. - PubMed
Jacobsen 2012 {published data only}
-
- Jacobsen PB, Wells KJ, Meade CD, Quinn GP, Lee J, Fulp WJ, et al. Effects of a brief multimedia psychoeducational intervention on the attitudes and interest of patients with cancer regarding clinical trial participation: a multicenter randomized controlled trial. Journal of Clinical Oncology 2012;30:2516‐21. - PMC - PubMed
Jimison 1998 {published data only}
Joseph 2006 {published data only}
-
- Joseph P, Schakman BR, Horwitz R, Nerette S, Verdier RI, Dorsainvil D, et al. The use of an educational video during informed consent in an HIV clinical trial in Haiti. Journal of Acquired Immune Deficiency Syndromes 2006;42(5):588‐91. - PubMed
Kim 2008 {published data only}
-
- Kim L, Young AJ, Neimeyer RA, Baker JN, Barfield R. Keeping users at the center: developing a multimedia interface for informed consent. Technical Communication Quarterly 2008;17:335‐57.
Llewellyn‐Thomas 1995 {published data only}
-
- Llewellyn‐Thomas HA, Thiel EC, Sem FWC, Harrison Woermke DE. Presenting clinical trial information: a comparison of methods. Patient Education & Counseling 1995;25:97‐107. - PubMed
McGraw 2012 {published data only}
-
- McGraw SA. Wood‐Nutter CA, Solomon MZ, Maschke KJ, Benspm JT, Irwin DE. Clarity and appeal of a multimedia informed consent tool for biobanking. IRB: Ethics & Human Research 2012;34:9‐19. - PubMed
Moseley 2006 {published data only}
Moser 2006 {published data only}
Palmer 2012 {published data only}
Quinn 2011 {published and unpublished data}
-
- Quinn G, Wells K, Antonia T, Antolino P, Lopez N, Gonzalez L, et al. Abstract A30: Evaluating Spanish audiovisual materials about cancer clinical trials: improving awareness among Hispanic patients and families. Cancer Epidemiology Biomarkers and Prevention. 2011; Vol. 20:np. [DOI: 10.1158/1055-9965.DISP-11-A30] - DOI
Sahai 2007 {published data only}
-
- Sahai A, Murphy D, Dasgupta P. Informed consent: are we deluding ourselves? A randomized controlled study. BJU International 2007;99:941. - PubMed
Simes 1986 {published data only}
Tindall 1994 {published data only}
-
- Tindall B, Forde S, Ross MW, Goldstein D, Barker S, Cooper DA. Effects of two formats of informed consent on knowledge among persons with advanced HIV disease in a clinical trial of didanosine. Patient Education and Counseling 1994;24:261‐6. - PubMed
Varnhagen 2005 {published data only}
-
- Varnhagen CK, Gushta M, Daniels J, Peters TC, Law D, Hirsch R, et al. How informed is online informed consent?. Ethics and Behaviour 2005;15(1):37‐48. - PubMed
Wells 2012 {published data only}
-
- Wells KJ, Quinn JP, Meade CD, Fletcher M, Martinez Tyson D, Jim H, et al. Development of a cancer clinical trials multi‐media intervention: Clinical trials: Are they right for you?. Patient Education and Counseling 2012;88:232‐40. - PubMed
Wragg 2000 {published data only}
-
- Wragg JA, Robinson EJ, Lilford RJ. Information presentation and decisions to enter clinical trials: a hypothetical trial of hormone replacement therapy. Social Science & Medicine 2000;51(3):453‐62. - PubMed
References to studies awaiting assessment
Hougham 2003b {published data only}
-
- Hougham GW, Sachs GA, Danner D, Mintz J, Patterson M, Roberts L, et al. University of California at Los Angeles study. Cited in Hougham 2003: study not cited by name, authors, only by affiliation.
Rowbotham 2013 {published data only}
Additional references
Agre 2003b
-
- Agre P, Campbell FA, Goldman BD, Boccia ML, Kass N, McCullough LB, et al. Improving informed consent: the medium is not the message. IRB: Ethics and Human Research (Supplement) 2003;25(5):S11‐19. - PubMed
Appelbaum 2010
-
- Appelbaum PS. Consent in impaired populations. Current Neurology and Neuroscience Reports 2010;10:367‐73. - PubMed
Ballard 2004
-
- Ballard HO, Shook LA, Desai NS, Anand KJ. Neonatal research and the validity of informed consent obtained in the perinatal period. Journal of Perinatology 2004;24(7):409‐15. - PubMed
Breese 2007
-
- Breese PE, Burman WJ, Goldberg S, Weis SE. Education level, primary language, and comprehension of the informed consent process. Journal of Empirical Research on Human Research Ethics 2007;2:69‐79. - PubMed
Brennan 2009
-
- Brennan S, McKenzie J, Whitty P, Buchan H, Green S. Continuous quality improvement: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003319.pub2] - DOI
Buchbinder 2004
-
- Buchbinder SP, Metch BMA, Holte SE, Scheer S, Coletti A, Vittinghoff E. Determinants of enrollment in a preventive HIV vaccine trial: hypothetical versus actual willingness and barriers to participation. Journal of Acquired Immune Deficiency Syndromes 2004;36:604‐12. - PubMed
Chan 2011
Chappuy 2006
Chuppay 2010
-
- Chappuy H, Baruchel A, Leverger G, Oudot C, Brethon B, Haouy S, et al. Parental comprehension and satisfaction in informed consent in paediatric clinical trials: a prospective study on childhood leukaemia. Archives of Disease in Childhood 2010;95:800‐4. - PubMed
Cohn 2007
-
- Cohn E, Larson E. Improving participant comprehension in the informed consent process. Journal of Nursing Scholarship 2007;39:273‐80. - PubMed
Dunn 2001a
-
- Dunn LB, Lindamer LA, Schneiderman LJ, Jeste DV. A novel, computer‐based enhancement of informed consent in older patients with psychotic disorders. 14th Annual Meeting of the American Association for Geriatric Psychiatry. San Francisco, CA, USA, 2001 23rd‐26th February.
Dunn 2006
-
- Dunn LB, Nowrangi MA, Palmer BW, Jeste DV, Saks ER. Assessing decisional capacity for clinical research or treatment: a review of instruments. American Journal of Psychiatry 2006;163(8):1323‐34. - PubMed
Edwards 2013
Enama 2012
-
- Enama ME, Hu Z, Gordon I, Costner P, Ledgerwood JE, Grady C and the VRC 306 and 307 Consent Study Teams. Randomization to standard and concise informed consent forms: development of evidence‐based consent practices. Contemporary Clinical Trials 2012;33:895‐902. [DOI: 10.1016/j.cct.2012.04.005] - DOI - PMC - PubMed
Fink 2010
-
- Fink AS, Prochazka AV, Henderson WG, Bartenfild D, Nyirenda C, Webb A, et al. Predictors of comprehension during surgical informed consent. Journal of the American College of Surgeons 2010;210:919‐26. - PubMed
Franck 2007
-
- Franck LS, Winter I, Oulton K. The quality of parental consent for research with children: a prospective repeated measure self‐report survey. International Journal of Nursing Studies 2007;44(4):525‐33. - PubMed
GRADEpro [Computer program]
-
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows 2008.
Guarino 2006
-
- Guarino P, Elbourne D, Carpenter J, Peduzzi P. Consumer involvement in consent document development: a multicenter cluster randomized trial to assess study participants' understanding. Clinical Trials 2006;3:19‐30. - PubMed
Guyatt 2008
Harth 1995
-
- Harth SC, Thong YH. Parental perceptions and attitudes about informed consent in clinical research involving children. Social Science & Medicine 1995;41(12):1647‐51. - PubMed
Hietanen 2000
-
- Hietanen P, Aro AR, Holli K, Absetz P. Information and communication in the context of a clinical trial. European Journal of Cancer 2000;36:2096‐104. - PubMed
Higgins 2011a
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, available from www.cochrane‐handbook.org 2011.
Higgins 2011b
-
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Joffe 2001a
-
- Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent in cancer clinical trials: a cross‐sectional survey. Lancet 2001;358:1772‐7. - PubMed
Joffe 2001b
-
- Joffe S, Cook EF, Cleary PD, Clark JW, Weeks JC. Quality of informed consent: a new measure of understanding among research subjects. Journal of the National Cancer Institute 2001;93(2):139‐47. - PubMed
Kinnersley 2013
-
- Kinnersley P, Phillips K, Savage K, Kelly MJ, Farrell E, Morgan B, et al. Interventions to promote informed consent for patients undergoing surgical and other invasive healthcare procedures. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD009445.pub2] - DOI - PMC - PubMed
Krosin 2006
-
- Krosin MT, Klitzman R, Levin B, Cheng J, Ranney ML. Problems in comprehension of informed consent in rural and peri‐urban Mali, West Africa. Clinical Trials 2006;3(3):306‐13. - PubMed
Kruse 2000
-
- Kruse AY, Kjaergard LL, Krogsgaard K, Gluud C, Mortensen EL, Gottschau A, et al. A randomized trial assessing the impact of written information on outpatients' knowlege about and attitude towards randomized clinical trials. Controlled Clinical Trials 2000;21(3):223‐40. - PubMed
Kupst 2003
-
- Kupst MJ, Patenaude AF, Walco GA, Sterling C. Clinical trials in pediatric cancer: parental perspectives on informed consent. Journal of Pediatric Hematology and Oncology 2003;25(10):787‐90. - PubMed
Miller 1996
-
- Miller CK, O’Donnell DC, Searight HR, Barbarash RA. The Deacones Informed Consent Comprehension Test: an assessment tool for clinical research subjects. Pharmacotherapy 1996;16:872‐8. - PubMed
Miller 2005
-
- Miller VA, Drotar D, Burant C, Kodish E. Clinician‐parent communication during informed consent for pediatric leukemia trials. Journal of Pediatric Psychology 2005;30(3):219‐29. - PubMed
Moodley 2005
NHMRC, ARC and AVCC 2007
-
- National Health and Medical Research Council, Australian Research Council and the Australian Vice‐Chancellor's Committee. National Statement on Ethical Conduct in Research. Australian Government, 2007.
NIH 2012
-
- NIH Clinical Research Trials and You. http://www.nih.gov/health/clinicaltrials/basics.htm 2012; Vol. accessed 4 March 2013.
Nilsen 2006
-
- Nilsen ES, Myrhaug HT, Johansen M, Oliver S, Oxman AD. Methods of consumer involvement in developing healthcare policy and research, clinical practice guidelines and patient information material. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004563.pub2] - DOI - PMC - PubMed
Nishimura 2013
Palmer 2005
-
- Palmer BW, Dunn LB, Appelbaum PS, Mudaliar S, Thal L, Henry R, et al. Assessment of capacity to consent to research among older persons with schizophrenia, Alzheimer's disease or diabetes mellitus. Archives of General Psychiatry 2005;62:726‐33. - PubMed
Pope 2003
-
- Pope JE, Tingey DP, Arnold JM, Hong P, Ouimet JM, Krizova A. Are subjects satisfied with the informed consent process? A survey of research participants. Journal of Rheumatology 2003;30(4):815‐24. - PubMed
Raffle 2001
Robinson 2005
-
- Robinson EJ, Kerr CEP, Stevens AJ, Lilford RJ, Braunholtz DA, Edwards SJ. Lay public's understanding of equipoise and randomisation in randomised controlled trials. Health Technology Assessment 2005; Vol. 9, issue 8. - PubMed
Ruccione 1991
-
- Ruccione K, Kramer RF, Moore IK, Perin G. Informed consent for treatment of childhood cancer: factors affecting parents' decision making. Journal of Pediatric Oncology Nursing 1991;8(3):112‐21. - PubMed
Ryan 2013
-
- Ryan R, Hill S, Prictor M, McKenzie J, Cochrane Consumers and Communication Review Group. Study Quality Guide. http://cccrg.cochrane.org/author‐resources (last accessed 5 Sep 2013) May 2013.
Schats 2003
Schünemann 2011
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration Available from www.cochrane‐handbook.org, 2011.
Sheridan 2011
-
- Sheridan SL, Halpern DJ, Viera AJ, Berkman ND, Donahue KE, Crotty K. Interventions for individuals with low health literacy: a systematic review. Journal of Health Communication: International Perspectives 2011;16:30‐54. - PubMed
Simon 2001
-
- Simon C, Eder M, Raiz P, Zyzanski S, Pentz R, Kodish ED. Informed consent for pediatric leukemia research: clinician perspectives. Cancer 2001;92(3):691‐700. - PubMed
Stacey 2014
Stanley 1998
-
- Stanley BM, Walters DJ, Maddern GJ. Informed consent: how much information is enough?. Australian and New Zealand Journal of Surgery 1998;68(11):788‐91. - PubMed
Stead 2005
Stryker 2006
-
- Stryker JE, Wray RJ, Emmons KM, Winer E, Demetri G. Understanding the decisions of cancer clinical trial participants to enter research studies: factors associated with informed consent, patient satisfaction, and decisional regret. Patient Education and Counseling 2006;63(1‐2):104‐9. - PubMed
Sugarman 1998
-
- Sugarman J, McCrory DC, Hubal RC. Getting meaningful informed consent from older adults: a structured literature review of empirical research. Journal of the American Geriatrics Society 1998;46:517‐24. - PubMed
Tait 2003
-
- Tait AR, Voepel‐Lewis T, Malviya S. Do they understand? (part I): parental consent for children participating in clinical anesthesia and surgery research. Anesthesiology 2003;98(3):603‐8. - PubMed
Treweek 2010
Verástegui 2006
WHO 2013
-
- World Health Organisation. International Clinical Trials Registry Platform (ICTRP). http://www.who.int/ictrp/en/ 2013, issue accessed 4 March 2013.
Williams 2003
-
- Williams, BF, French JK, White HD. Informed consent during the clinical emergency of acute myocardial infarction (HERO‐2 consent substudy): a prospective observational study. Lancet 2003;361(9361):918‐22. - PubMed
WMA 2008
-
- World Medical Association Declaration of Helsinki. Ethical Principles for Medical Research Involving Human Subjects. Adopted by the 18th WMA General Assembly Helsinki, Finland, June 1964 and amended by the 29th WMA General Assembly, Tokyo, Japan, October 1975, 35th WMA General Assembly, Venice, Italy, October 1983, 41st WMA General Assembly, Hong Kong, September 1989, 48th WMA General Assembly, Somerset West, Republic of South Africa, October 1996, 52nd WMA General Assembly, Edinburgh, Scotland, October 2000, 53rd WMA General Assembly, Washington, DC, USA, October 2002, 55th WMA General Assembly, Tokyo, Japan, October 2004 and 59th WMA General Assembly, Seoul, Korea, October 2008.
References to other published versions of this review
McLaughlin 2002
-
- McLaughlin KJ, Brindley AF, Crowther CA. Informational video for potential participants of clinical studies used in the process of seeking informed consent. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003717] - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

