Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP)
- PMID: 24810037
- PMCID: PMC4102313
- DOI: 10.1038/nprot.2014.085
Cell type-specific mRNA purification by translating ribosome affinity purification (TRAP)
Abstract
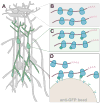
Cellular diversity and architectural complexity create barriers to understanding the function of the mammalian CNS at a molecular level. To address this problem, we have recently developed a methodology that provides the ability to profile the entire translated mRNA complement of any genetically defined cell population. This methodology, which we termed translating ribosome affinity purification, or TRAP, combines cell type-specific transgene expression with affinity purification of translating ribosomes. TRAP can be used to study the cell type-specific mRNA profiles of any genetically defined cell type, and it has been used in organisms ranging from Drosophila melanogaster to mice and human cultured cells. Unlike other methodologies that rely on microdissection, cell panning or cell sorting, the TRAP methodology bypasses the need for tissue fixation or single-cell suspensions (and the potential artifacts that these treatments introduce) and reports on mRNAs in the entire cell body. This protocol provides a step-by-step guide to implement the TRAP methodology, which takes 2 d to complete once all materials are in hand.
Conflict of interest statement
The authors have no competing financial interests.
Figures

Similar articles
-
TRAP-rc, Translating Ribosome Affinity Purification from Rare Cell Populations of Drosophila Embryos.J Vis Exp. 2015 Sep 10;(103):52985. doi: 10.3791/52985. J Vis Exp. 2015. PMID: 26381166 Free PMC article.
-
Translating Ribosome Affinity Purification (TRAP) followed by RNA sequencing technology (TRAP-SEQ) for quantitative assessment of plant translatomes.Methods Mol Biol. 2015;1284:185-207. doi: 10.1007/978-1-4939-2444-8_9. Methods Mol Biol. 2015. PMID: 25757773
-
Translating ribosome affinity purification (TRAP) for cell-specific translation profiling in developing flowers.Methods Mol Biol. 2014;1110:323-8. doi: 10.1007/978-1-4614-9408-9_18. Methods Mol Biol. 2014. PMID: 24395267
-
Translatome profiling: methods for genome-scale analysis of mRNA translation.Brief Funct Genomics. 2016 Jan;15(1):22-31. doi: 10.1093/bfgp/elu045. Epub 2014 Nov 6. Brief Funct Genomics. 2016. PMID: 25380596 Review.
-
Profiling translation in the nervous system.J Biochem. 2025 Apr 2;177(4):239-246. doi: 10.1093/jb/mvae096. J Biochem. 2025. PMID: 39745834 Review.
Cited by
-
Cell Type-specific mRNA Purification in Caenorhabditis elegans via Translating Ribosome Affinity Purification.Bio Protoc. 2019 Aug 5;9(15):e3328. doi: 10.21769/BioProtoc.3328. eCollection 2019 Aug 5. Bio Protoc. 2019. PMID: 33654835 Free PMC article.
-
Bridging the Gap: Towards a cell-type specific understanding of neural circuits underlying fear behaviors.Neurobiol Learn Mem. 2016 Nov;135:27-39. doi: 10.1016/j.nlm.2016.07.025. Epub 2016 Jul 26. Neurobiol Learn Mem. 2016. PMID: 27470092 Free PMC article. Review.
-
Cell cycle specific, differentially tagged ribosomal proteins to measure phase specific transcriptomes from asynchronously cycling cells.Sci Rep. 2024 Jan 18;14(1):1623. doi: 10.1038/s41598-024-52085-5. Sci Rep. 2024. PMID: 38238470 Free PMC article.
-
RPL-4 and RPL-9 ̶Mediated Ribosome Purifications Facilitate the Efficient Analysis of Gene Expression in Caenorhabditis elegans Germ Cells.G3 (Bethesda). 2020 Nov 5;10(11):4063-4069. doi: 10.1534/g3.120.401644. G3 (Bethesda). 2020. PMID: 32883755 Free PMC article.
-
Recent progress in single-molecule studies of mRNA localization in vivo.RNA Biol. 2019 Sep;16(9):1108-1118. doi: 10.1080/15476286.2018.1536592. Epub 2018 Nov 14. RNA Biol. 2019. PMID: 30336727 Free PMC article. Review.
References
-
- Wang S, Roy NS, Benraiss A, Goldman SA. Promoter-based isolation and fluorescence-activated sorting of mitotic neuronal progenitor cells from the adult mammalian ependymal/subependymal zone. Developmental neuroscience. 2000;22:167–176. 17437. - PubMed
-
- Tomomura M, Rice DS, Morgan JI, Yuzaki M. Purification of Purkinje cells by fluorescence-activated cell sorting from transgenic mice that express green fluorescent protein. The European journal of neuroscience. 2001;14:57–63. - PubMed
Key references
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

