Celastrol targets proteostasis and acts synergistically with a heat-shock protein 90 inhibitor to kill human glioblastoma cells
- PMID: 24810052
- PMCID: PMC4047902
- DOI: 10.1038/cddis.2014.182
Celastrol targets proteostasis and acts synergistically with a heat-shock protein 90 inhibitor to kill human glioblastoma cells
Abstract
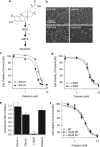
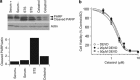
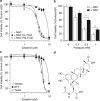
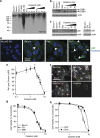
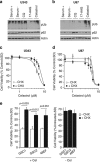
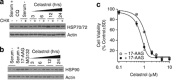
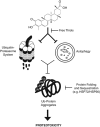
Glioblastoma multiforme is a devastating disease of the central nervous system and, at present, no effective therapeutic interventions have been identified. Celastrol, a natural occurring triterpene, exhibits potent anti-tumor activity against gliomas in xenograft mouse models. In this study, we describe the cell death mechanism employed by celastrol and identify secondary targets for effective combination therapy against glioblastoma cell survival. In contrast to the previously proposed reactive oxygen species (ROS)-dependent mechanism, cell death in human glioblastoma cells is shown here to be mediated by alternate signal transduction pathways involving, but not fully dependent on, poly(ADP-ribose) polymerase-1 and caspase-3. Our studies indicate that celastrol promotes proteotoxic stress, supported by two feedback mechanisms: (i) impairment of protein quality control as revealed by accumulation of polyubiquitinated aggregates and the canonical autophagy substrate, p62, and (ii) the induction of heat-shock proteins, HSP72 and HSP90. The Michael adduct of celastrol and N-acetylcysteine, 6-N-acetylcysteinyldihydrocelastrol, had no effect on p62, nor on HSP72 expression, confirming a thiol-dependent mechanism. Restriction of protein folding stress with cycloheximide was protective, while combination with autophagy inhibitors did not sensitize cells to celastrol-mediated cytotoxicity. Collectively, these findings imply that celastrol targets proteostasis by disrupting sulfyhydryl homeostasis, independently of ROS, in human glioblastoma cells. This study further emphasizes that targeting proteotoxic stress responses by inhibiting HSP90 with 17-N-Allylamino-17-demethoxygeldanamycin sensitizes human glioblastoma to celastrol treatment, thereby serving as a novel synergism to overcome drug resistance.
Figures
References
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Lima FR, Kahn SA, Soletti RC, Biasoli D, Alves T, da Fonseca AC, et al. Glioblastoma: therapeutic challenges, what lies ahead. Biochim Biophys Acta. 2012;1826:338–349. - PubMed
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Wang Y, Jiang T. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331:139–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

