Mitochondrial dysfunction driven by the LRRK2-mediated pathway is associated with loss of Purkinje cells and motor coordination deficits in diabetic rat model
- PMID: 24810053
- PMCID: PMC4047887
- DOI: 10.1038/cddis.2014.184
Mitochondrial dysfunction driven by the LRRK2-mediated pathway is associated with loss of Purkinje cells and motor coordination deficits in diabetic rat model
Abstract
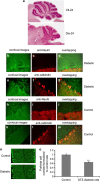
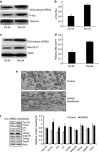
Diabetic neuropathy develops on a background of hyperglycemia and an entangled metabolic imbalance. There is increasing evidence of central nervous system involvement in diabetic neuropathy and no satisfactory treatment except maintenance of good glycemic control, thereby highlighting the importance of identifying novel therapeutic targets. Purkinje cells are a class of metabolically specialized active neurons, and degeneration of Purkinje cells is a common feature of inherited ataxias in humans and mice. However, whether Purkinje cells are implicated in diabetic neuropathy development under metabolic stress remains poorly defined. Here, we revealed a novel leucine-rich repeat kinase 2 (LRRK2)-mediated pathway in Purkinje cells that is involved in the pathogenesis of diabetic neuropathy from a 24-week long study of streptozotocin (STZ)-diabetic rats. We found that hyperglycemia, cerebellum proinflammatory cytokines, and chemokines increased markedly in 24-week STZ-diabetic rats. Furthermore, we demonstrated that degeneration of Purkinje cells is characterized by progressive swellings of axon terminals, no autophagosome formation, the reduction of LC3II/LC3I and Lamp2, and accumulation of p62 puncta in 24-week STZ-diabetic rats. Importantly, a higher expression level of LRRK2-mediated hyperphosphorylation of tau along with increased mitochondrial dynamin-like protein (mito-DLP1) was demonstrated in 24-week STZ-diabetic rats. This effect of LRRK2 overexpression induced mitochondrial fragmentation, and reduced mitochondrial protein degradation rates were confirmed in vitro. As a consequence, 24-week STZ-diabetic rats showed mitochondrial dysfunction in cerebellar Purkinje neurons and coordinated motor deficits evaluated by rotarod test. Our findings are to our knowledge the first to suggest that the LRRK2-mediated pathway induces mitochondrial dysfunction and loss of cerebellar Purkinje neurons and, subsequently, may be associated with motor coordination deficits in STZ-diabetic rats. These data may indicate a novel cellular therapeutic target for diabetic neuropathy.
Figures





Similar articles
-
LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1.Hum Mol Genet. 2012 May 1;21(9):1931-44. doi: 10.1093/hmg/dds003. Epub 2012 Jan 6. Hum Mol Genet. 2012. PMID: 22228096 Free PMC article.
-
Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via Dynamin-like protein.J Neurochem. 2012 Aug;122(3):650-8. doi: 10.1111/j.1471-4159.2012.07809.x. Epub 2012 Jun 22. J Neurochem. 2012. PMID: 22639965
-
Insulin therapy modulates mitochondrial dynamics and biogenesis, autophagy and tau protein phosphorylation in the brain of type 1 diabetic rats.Biochim Biophys Acta. 2014 Jul;1842(7):1154-66. doi: 10.1016/j.bbadis.2014.04.011. Epub 2014 Apr 18. Biochim Biophys Acta. 2014. PMID: 24747740
-
Nutrient excess and altered mitochondrial proteome and function contribute to neurodegeneration in diabetes.Mitochondrion. 2011 Nov;11(6):845-54. doi: 10.1016/j.mito.2011.06.007. Epub 2011 Jul 2. Mitochondrion. 2011. PMID: 21742060 Free PMC article. Review.
-
Different Types of Cell Death in Diabetic Neuropathy: A Focus on Mechanisms and Therapeutic Strategies.Int J Mol Sci. 2024 Jul 25;25(15):8126. doi: 10.3390/ijms25158126. Int J Mol Sci. 2024. PMID: 39125694 Free PMC article. Review.
Cited by
-
Opposite Roles of Co-enzyme Q10 and Formaldehyde in Neurodegenerative Diseases.Am J Alzheimers Dis Other Demen. 2022 Jan-Dec;37:15333175221143274. doi: 10.1177/15333175221143274. Am J Alzheimers Dis Other Demen. 2022. PMID: 36455136 Free PMC article. Review.
-
Peroxiredoxin 1 inhibits streptozotocin-induced Alzheimer's disease-like pathology in hippocampal neuronal cells via the blocking of Ca2+/Calpain/Cdk5-mediated mitochondrial fragmentation.Sci Rep. 2024 Jul 8;14(1):15642. doi: 10.1038/s41598-024-66256-x. Sci Rep. 2024. PMID: 38977865 Free PMC article.
-
Induced pluripotent stem cell-based modeling of mutant LRRK2-associated Parkinson's disease.Eur J Neurosci. 2019 Feb;49(4):561-589. doi: 10.1111/ejn.14345. Eur J Neurosci. 2019. PMID: 30656775 Free PMC article. Review.
-
Gene Therapeutic Approaches for the Treatment of Mitochondrial Dysfunction in Parkinson's Disease.Genes (Basel). 2021 Nov 22;12(11):1840. doi: 10.3390/genes12111840. Genes (Basel). 2021. PMID: 34828446 Free PMC article. Review.
-
LRRK2 maintains mitochondrial homeostasis and regulates innate immune responses to Mycobacterium tuberculosis.Elife. 2020 Feb 14;9:e51071. doi: 10.7554/eLife.51071. Elife. 2020. PMID: 32057291 Free PMC article.
References
-
- Roman de Mettelinge T, Delbaere K, Calders P, Gysel T, Van Den Noortgate N, Cambier D. The impact of peripheral neuropathy and cognitive decrements on gait in older adults with type 2 diabetes mellitus. Arch Phys Med Rehabil. 2013;94:1074–1079. - PubMed
-
- Di Domenico F, Sultana R, Ferree A, Smith K, Barone E, Perluigi M, et al. Redox proteomics analyses of the influence of co-expression of wild-type or mutated LRRK2 and Tau on C. elegans protein expression and oxidative modification: relevance to Parkinson disease. Antioxid Redox Signal. 2012;17:1490–1506. - PMC - PubMed
-
- Miklossy J, Qing H, Guo JP, Yu S, Wszolek ZK, Calne D, et al. Lrrk2 and chronic inflammation are linked to pallido-ponto-nigral degeneration caused by the N279K tau mutation. Acta Neuropathol. 2007;114:243–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

