Zeaxanthin induces Nrf2-mediated phase II enzymes in protection of cell death
- PMID: 24810054
- PMCID: PMC4047913
- DOI: 10.1038/cddis.2014.190
Zeaxanthin induces Nrf2-mediated phase II enzymes in protection of cell death
Abstract
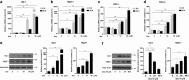
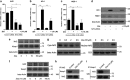
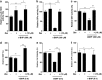
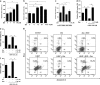
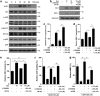
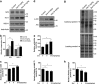
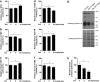
Zeaxanthin (Zea) is a major carotenoid pigment contained in human retina, and its daily supplementation associated with lower risk of age-related macular degeneration. Despite known property of Zea as an antioxidant, its underlying molecular mechanisms of action remain poorly understood. In this study, we aim to study the regulation mechanism of Zea on phase II detoxification enzymes. In normal human retinal pigment epithelium cells, Zea promoted the nuclear translocation of NF-E2-related factor 2 (Nrf2) and induced mRNA and protein expression of phase II enzymes, the induction was suppressed by specific knockdown of Nrf2. Zea also effectively protected against tert-butyl hydroperoxide-induced mitochondrial dysfunction and apoptosis. Glutathione (GSH) as the most important antioxidant was also induced by Zea through Nrf2 activation in a time- and dose-dependent manner, whereas the protective effects of Zea were decimated by inhibition of GSH synthesis. Finally, Zea activated the PI3K/Akt and MAPK/ERK pathway, whereas only PI3K/Akt activation correlated with phase II enzymes induction and Zea protection. In further in vivo analyses, Zea showed effects of inducing phase II enzymes and increased GSH content, which contributed to the reduced lipid and protein peroxidation in the retina as well as the liver, heart, and serum of the Sprague-Dawley rats. For the first time, Zea is presented as a phase II enzymes inducer instead of being an antioxidant. By activating Nrf2-mediated phase II enzymes, Zea could enhance anti-oxidative capacity and prevent cell death both in vivo and in vitro.
Figures


Similar articles
-
Astaxanthin protects ARPE-19 cells from oxidative stress via upregulation of Nrf2-regulated phase II enzymes through activation of PI3K/Akt.Mol Vis. 2013 Jul 25;19:1656-66. Print 2013. Mol Vis. 2013. PMID: 23901249 Free PMC article.
-
Hydroxytyrosol induces antioxidant/detoxificant enzymes and Nrf2 translocation via extracellular regulated kinases and phosphatidylinositol-3-kinase/protein kinase B pathways in HepG2 cells.Mol Nutr Food Res. 2010 Jul;54(7):956-66. doi: 10.1002/mnfr.200900159. Mol Nutr Food Res. 2010. PMID: 20166143
-
Activation of Akt and JNK/Nrf2/NQO1 pathway contributes to the protective effect of coptisine against AAPH-induced oxidative stress.Biomed Pharmacother. 2017 Jan;85:313-322. doi: 10.1016/j.biopha.2016.11.031. Epub 2016 Nov 27. Biomed Pharmacother. 2017. PMID: 27903425
-
Piceatannol Protects Human Retinal Pigment Epithelial Cells against Hydrogen Peroxide Induced Oxidative Stress and Apoptosis through Modulating PI3K/Akt Signaling Pathway.Nutrients. 2019 Jul 4;11(7):1515. doi: 10.3390/nu11071515. Nutrients. 2019. PMID: 31277394 Free PMC article.
-
Xanthophylls.Adv Nutr. 2018 Mar 1;9(2):160-162. doi: 10.1093/advances/nmx005. Adv Nutr. 2018. PMID: 29659682 Free PMC article. Review. No abstract available.
Cited by
-
4-Acetoxyphenol Prevents RPE Oxidative Stress-Induced Necrosis by Functioning as an NRF2 Stabilizer.Invest Ophthalmol Vis Sci. 2015 Aug;56(9):5048-59. doi: 10.1167/iovs.15-16401. Invest Ophthalmol Vis Sci. 2015. PMID: 26241392 Free PMC article.
-
Anti-Inflammatory and Anticancer Effects of Microalgal Carotenoids.Mar Drugs. 2021 Sep 23;19(10):531. doi: 10.3390/md19100531. Mar Drugs. 2021. PMID: 34677429 Free PMC article. Review.
-
Protective Effect of a Water-Soluble Carotenoid-Rich Extract of Cordyceps militaris against Light-Evoked Functional Vision Deterioration in Mice.Nutrients. 2022 Apr 18;14(8):1675. doi: 10.3390/nu14081675. Nutrients. 2022. PMID: 35458237 Free PMC article.
-
Microalgae as a Nutraceutical Tool to Antagonize the Impairment of Redox Status Induced by SNPs: Implications on Insulin Resistance.Biology (Basel). 2023 Mar 15;12(3):449. doi: 10.3390/biology12030449. Biology (Basel). 2023. PMID: 36979141 Free PMC article. Review.
-
Non-invasive pharmacological advances in early retinopathy treatment: bioactive herbal compounds, polymer delivery systems, and computational bioprospecting of functional targets.Pharmacol Rep. 2025 Aug 28. doi: 10.1007/s43440-025-00778-7. Online ahead of print. Pharmacol Rep. 2025. PMID: 40866756 Review.
References
-
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999;274:26071–26078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

