Partial agonist, telmisartan, maintains PPARγ serine 112 phosphorylation, and does not affect osteoblast differentiation and bone mass
- PMID: 24810249
- PMCID: PMC4014504
- DOI: 10.1371/journal.pone.0096323
Partial agonist, telmisartan, maintains PPARγ serine 112 phosphorylation, and does not affect osteoblast differentiation and bone mass
Abstract
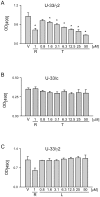
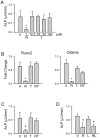
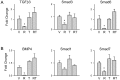
Peroxisome proliferator activated receptor gamma (PPARγ) controls both glucose metabolism and an allocation of marrow mesenchymal stem cells (MSCs) toward osteoblast and adipocyte lineages. Its activity is determined by interaction with a ligand which directs posttranscriptional modifications of PPARγ protein including dephosphorylation of Ser112 and Ser273, which results in acquiring of pro-adipocytic and insulin-sensitizing activities, respectively. PPARγ full agonist TZD rosiglitazone (ROSI) decreases phosphorylation of both Ser112 and Ser273 and its prolonged use causes bone loss in part due to diversion of MSCs differentiation from osteoblastic toward adipocytic lineage. Telmisartan (TEL), an anti-hypertensive drug from the class of angiotensin receptor blockers, also acts as a partial PPARγ agonist with insulin-sensitizing and a weak pro-adipocytic activity. TEL decreased S273pPPARγ and did not affect S112pPPARγ levels in a model of marrow MSC differentiation, U-33/γ2 cells. In contrast to ROSI, TEL did not affect osteoblast phenotype and actively blocked ROSI-induced anti-osteoblastic activity and dephosphorylation of S112pPPARγ. The effect of TEL on bone was tested side-by-side with ROSI. In contrast to ROSI, TEL administration did not affect bone mass and bone biomechanical properties measured by micro-indentation method and did not induce fat accumulation in bone, and it partially protected from ROSI-induced bone loss. In addition, TEL induced "browning" of epididymal white adipose tissue marked by increased expression of UCP1, FoxC2, Wnt10b and IGFBP2 and increased overall energy expenditure. These studies point to the complexity of mechanisms by which PPARγ acquires anti-osteoblastic and pro-adipocytic activities and suggest an importance of Ser112 phosphorylation status as being a part of the mechanism regulating this process. These studies showed that TEL acts as a full PPARγ agonist for insulin-sensitizing activity and as a partial agonist/partial antagonist for pro-adipocytic and anti-osteoblastic activities. They also suggest a relationship between PPARγ fat "browning" activity and a lack of anti-osteoblastic activity.
Conflict of interest statement
Figures




Similar articles
-
β-catenin directly sequesters adipocytic and insulin sensitizing activities but not osteoblastic activity of PPARγ2 in marrow mesenchymal stem cells.PLoS One. 2012;7(12):e51746. doi: 10.1371/journal.pone.0051746. Epub 2012 Dec 18. PLoS One. 2012. PMID: 23272157 Free PMC article.
-
Protein Phosphatase PP5 Controls Bone Mass and the Negative Effects of Rosiglitazone on Bone through Reciprocal Regulation of PPARγ (Peroxisome Proliferator-activated Receptor γ) and RUNX2 (Runt-related Transcription Factor 2).J Biol Chem. 2016 Nov 18;291(47):24475-24486. doi: 10.1074/jbc.M116.752493. Epub 2016 Sep 29. J Biol Chem. 2016. Retraction in: J Biol Chem. 2018 May 25;293(21):8314. doi: 10.1074/jbc.W118.003815. PMID: 27687725 Free PMC article. Retracted.
-
Netoglitazone is a PPAR-gamma ligand with selective effects on bone and fat.Bone. 2006 Jan;38(1):74-84. doi: 10.1016/j.bone.2005.07.008. Epub 2005 Aug 30. Bone. 2006. PMID: 16137931 Free PMC article.
-
The role of PPARγ for the osteoblastic differentiation.J Endocrinol Invest. 2010;33(7 Suppl):9-12. J Endocrinol Invest. 2010. PMID: 20938219 Review.
-
Treating the metabolic syndrome: telmisartan as a peroxisome proliferator-activated receptor-gamma activator.Acta Diabetol. 2005 Apr;42 Suppl 1:S9-16. doi: 10.1007/s00592-005-0176-0. Acta Diabetol. 2005. PMID: 15868121 Review.
Cited by
-
Effects of telmisartan and losartan treatments on bone turnover markers in patients with newly diagnosed stage I hypertension.J Renin Angiotensin Aldosterone Syst. 2019 Jul-Sep;20(3):1470320319862741. doi: 10.1177/1470320319862741. J Renin Angiotensin Aldosterone Syst. 2019. PMID: 31328615 Free PMC article. Clinical Trial.
-
The Antihypertensive Drug Telmisartan Protects Oligodendrocytes from Cholesterol Accumulation and Promotes Differentiation by a PPAR-γ-Mediated Mechanism.Int J Mol Sci. 2021 Aug 30;22(17):9434. doi: 10.3390/ijms22179434. Int J Mol Sci. 2021. PMID: 34502342 Free PMC article.
-
The Role of Peroxisome Proliferator-Activated Receptor Gamma and Atherosclerosis: Post-translational Modification and Selective Modulators.Front Physiol. 2022 Mar 2;13:826811. doi: 10.3389/fphys.2022.826811. eCollection 2022. Front Physiol. 2022. PMID: 35309069 Free PMC article. Review.
-
The Embryonic Chick Femur Organotypic Model as a Tool to Analyze the Angiotensin II Axis on Bone Tissue.Pharmaceuticals (Basel). 2021 May 16;14(5):469. doi: 10.3390/ph14050469. Pharmaceuticals (Basel). 2021. PMID: 34065702 Free PMC article.
-
Marrow Fat-a New Target to Treat Bone Diseases?Curr Osteoporos Rep. 2018 Apr;16(2):123-129. doi: 10.1007/s11914-018-0426-z. Curr Osteoporos Rep. 2018. PMID: 29460176 Review.
References
-
- Tontonoz P, Spiegelman BM (2008) Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem 77: 289–312. - PubMed
-
- Lecka-Czernik B, Gubrij I, Moerman EA, Kajkenova O, Lipschitz DA, et al. (1999) Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPAR-gamma 2. J Cell Biochem 74: 357–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

