Berberine exposure triggers developmental effects on planarian regeneration
- PMID: 24810466
- PMCID: PMC4014983
- DOI: 10.1038/srep04914
Berberine exposure triggers developmental effects on planarian regeneration
Abstract
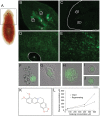
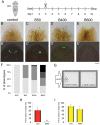
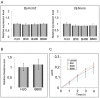
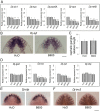
The mechanisms of action underlying the pharmacological properties of the natural alkaloid berberine still need investigation. Planarian regeneration is instrumental in deciphering developmental responses following drug exposure. Here we report the effects of berberine on regeneration in the planarian Dugesia japonica. Our findings demonstrate that this compound perturbs the regenerative pattern. By real-time PCR screening for the effects of berberine exposure on gene expression, we identified alterations in the transcriptional profile of genes representative of different tissues, as well as of genes involved in extracellular matrix (ECM) remodeling. Although berberine does not influence cell proliferation/apoptosis, our experiments prove that this compound causes abnormal regeneration of the planarian visual system. Potential berberine-induced cytotoxic effects were noticed in the intestine. Although we were unable to detect abnormalities in other structures, our findings, sustained by RNAi-based investigations, support the possibility that berberine effects are critically linked to anomalous ECM remodeling in treated planarians.
Figures

References
-
- Gan R. Y. Bioactivities of Berberine: An Update. Int. J. Modern Biol. Med. 1, 48–81 (2012).
-
- Gilca M., Gaman L., Panait E., Stoian I. & Atanasiu V. Chelidonium majus: an integrative review: traditional knowledge versus modern findings. Forsch. Komplementmed. 17, 241–248 (2010). - PubMed
-
- Isolani M. E. et al. The in vivo effect of chelidonine on the stem cell system of planarians. Eur. J. Pharmacol. 686, 1–3 (2012). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

