Field-evaluation of a new lateral flow assay for detection of cellular and humoral immunity against Mycobacterium leprae
- PMID: 24810599
- PMCID: PMC4014418
- DOI: 10.1371/journal.pntd.0002845
Field-evaluation of a new lateral flow assay for detection of cellular and humoral immunity against Mycobacterium leprae
Abstract
Background: Field-applicable tests detecting asymptomatic Mycobacterium leprae (M. leprae) infection or predicting progression to leprosy, are urgently required. Since the outcome of M. leprae infection is determined by cellular- and humoral immunity, we aim to develop diagnostic tests detecting pro-/anti-inflammatory and regulatory cytokines as well as antibodies against M. leprae. Previously, we developed lateral flow assays (LFA) for detection of cytokines and anti-PGL-I antibodies. Here we evaluate progress of newly developed LFAs for applications in resource-poor settings.
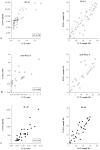
Methods: The combined diagnostic value of IP-10, IL-10 and anti-PGL-I antibodies was tested using M. leprae-stimulated blood of leprosy patients and endemic controls (EC). For reduction of the overall test-to-result time the minimal whole blood assay time required to detect distinctive responses was investigated. To accommodate LFAs for field settings, dry-format LFAs for IP-10 and anti-PGL-I antibodies were developed allowing storage and shipment at ambient temperatures. Additionally, a multiplex LFA-format was applied for simultaneous detection of anti-PGL-I antibodies and IP-10. For improved sensitivity and quantitation upconverting phosphor (UCP) reporter technology was applied in all LFAs.
Results: Single and multiplex UCP-LFAs correlated well with ELISAs. The performance of dry reagent assays and portable, lightweight UCP-LF strip readers indicated excellent field-robustness. Notably, detection of IP-10 levels in stimulated samples allowed a reduction of the whole blood assay time from 24 h to 6 h. Moreover, IP-10/IL-10 ratios in unstimulated plasma differed significantly between patients and EC, indicating the feasibility to identify M. leprae infection in endemic areas.
Conclusions: Dry-format UCP-LFAs are low-tech, robust assays allowing detection of relevant cytokines and antibodies in response to M. leprae in the field. The high levels of IP-10 and the required shorter whole blood assay time, render this cytokine useful to discriminate between leprosy patients and EC.
Conflict of interest statement
The authors have declared that no competing interest exist.
Figures





References
-
- Global leprosy: update on the 2012 situation. Wkly Epidemiol Rec 88: 365–379. - PubMed
-
- Ridley DS, Jopling WH (1966) Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis 34: 255–273. - PubMed
-
- Burki T (2010) Fight against leprosy no longer about the numbers. Lancet Infect Dis 10: 74. - PubMed
-
- Niedbala RS, Feindt H, Kardos K, Vail T, Burton J, et al. (2001) Detection of analytes by immunoassay using up-converting phosphor technology. Anal Biochem 293: 22–30. - PubMed
-
- Qu Q, Zhu Z, Wang Y, Zhong Z, Zhao J, et al. (2009) Rapid and quantitative detection of Brucella by up-converting phosphor technology-based lateral-flow assay. J Microbiol Methods 79: 121–123 S0167-7012(09)00228-0 [pii];10.1016/j.mimet.2009.07.015 [doi] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

