A PrP(C)-caveolin-Lyn complex negatively controls neuronal GSK3β and serotonin 1B receptor
- PMID: 24810941
- PMCID: PMC4013941
- DOI: 10.1038/srep04881
A PrP(C)-caveolin-Lyn complex negatively controls neuronal GSK3β and serotonin 1B receptor
Abstract
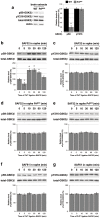
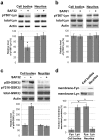
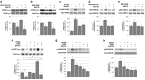
The cellular prion protein, PrP(C), is a glycosylphosphatidylinositol-anchored protein, abundant in lipid rafts and highly expressed in the brain. While PrP(C) is much studied for its involvement under its abnormal PrP(Sc) isoform in Transmissible Spongiform Encephalopathies, its physiological role remains unclear. Here, we report that GSK3β, a multifunctional kinase whose inhibition is neuroprotective, is a downstream target of PrP(C) signalling in serotonergic neuronal cells. We show that the PrP(C)-dependent inactivation of GSK3β is relayed by a caveolin-Lyn platform located on neuronal cell bodies. Furthermore, the coupling of PrP(C) to GSK3β potentiates serotonergic signalling by altering the distribution and activity of the serotonin 1B receptor (5-HT1BR), a receptor that limits neurotransmitter release. In vivo, our data reveal an increased GSK3β kinase activity in PrP-deficient mouse brain, as well as sustained 5-HT1BR activity, whose inhibition promotes an anxiogenic behavioural response. Collectively, our data unveil a new facet of PrP(C) signalling that strengthens neurotransmission.
Figures




References
-
- Aguzzi A. & Calella A. M. Prions: protein aggregation and infectious diseases. Physiol Rev. 89, 1105–1152 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

