WT1 regulates angiogenesis in Ewing Sarcoma
- PMID: 24810959
- PMCID: PMC4058017
- DOI: 10.18632/oncotarget.1610
WT1 regulates angiogenesis in Ewing Sarcoma
Abstract
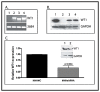
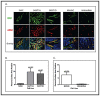
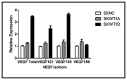
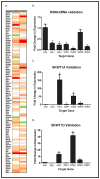
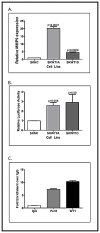
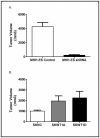
Angiogenesis is required for tumor growth. WT1, a protein that affects both mRNA transcription and splicing, has recently been shown to regulate expression of vascular endothelial growth factor (VEGF), one of the major mediators of angiogenesis. In the present study, we tested the hypothesis that WT1 is a key regulator of tumor angiogenesis in Ewing sarcoma. We expressed exogenous WT1 in the WT1-null Ewing sarcoma cell line, SK-ES-1, and we suppressed WT1 expression using shRNA in the WT1-positive Ewing sarcoma cell line, MHH-ES. Suppression of WT1 in MHH-ES cells impairs angiogenesis, while expression of WT1 in SK-ES-1 cells causes increased angiogenesis. Different WT1 isoforms result in vessels with distinct morphologies, and this correlates with preferential upregulation of particular VEGF isoforms. WT1-expressing tumors show increased expression of pro-angiogenic molecules such as VEGF, MMP9, Ang-1, and Tie-2, supporting the hypothesis that WT1 is a global regulator of angiogenesis. We also demonstrate that WT1 regulates the expression of a panel of pro-angiogenic molecules in Ewing sarcoma cell lines. Finally, we found that WT1 expression is correlated with VEGF expression, MMP9 expression, and microvessel density in samples of primary Ewing sarcoma. Thus, our results demonstrate that WT1 expression directly regulates tumor angiogenesis by controlling the expression of a panel of pro-angiogenic genes.
Figures


References
-
- Carpentieri DF, Nichols K, Chou PM, Matthews M, Pawel B, Huff D. The expression of WT1 in the differentiation of rhabdomyosarcoma from other pediatric small round blue cell tumors. Mod Pathol. 2002;15(10):1080–1086. - PubMed
-
- Inoue K, Ogawa H, Sonoda Y, Kimura T, Sakabe H, Oka Y, Miyake S, Tamaki H, Oji Y, Yamagami T, Tatekawa T, Soma T, Kishimoto T, Sugiyama H. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukemia. Blood. 1997;89(4):1405–1412. - PubMed
-
- Loeb DM, Evron E, Patel CB, Sharma PM, Niranjan B, Buluwela L, Weitzman SA, Korz D, Sukumar S. Wilms' tumor suppressor gene (WT1) is expressed in primary breast tumors despite tumor-specific promoter methylation. Cancer Res. 2001;61(3):921–925. - PubMed
-
- Miwa H, Beran M, Saunders GF. Expression of the Wilms' tumor gene (WT1) in human leukemias. Leukemia. 1992;6(5):405–409. - PubMed
-
- Miyoshi Y, Ando A, Egawa C, Taguchi T, Tamaki Y, Tamaki H, Sugiyama H, Noguchi S. High expression of Wilms' tumor suppressor gene predicts poor prognosis in breast cancer patients. Clin Cancer Res. 2002;8(5):1167–1171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

