A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival
- PMID: 24811110
- PMCID: PMC4076028
- DOI: 10.1021/nn5014484
A nanoparticle carrying the p53 gene targets tumors including cancer stem cells, sensitizes glioblastoma to chemotherapy and improves survival
Abstract
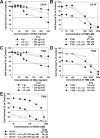
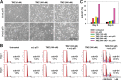
Temozolomide (TMZ)-resistance in glioblastoma multiforme (GBM) has been linked to upregulation of O(6)-methylguanine-DNA methyltransferase (MGMT). Wild-type (wt) p53 was previously shown to down-modulate MGMT. However, p53 therapy for GBM is limited by lack of efficient delivery across the blood brain barrier (BBB). We have developed a systemic nanodelivery platform (scL) for tumor-specific targeting (primary and metastatic), which is currently in multiple clinical trials. This self-assembling nanocomplex is formed by simple mixing of the components in a defined order and a specific ratio. Here, we demonstrate that scL crosses the BBB and efficiently targets GBM, as well as cancer stem cells (CSCs), which have been implicated in recurrence and treatment resistance in many human cancers. Moreover, systemic delivery of scL-p53 down-modulates MGMT and induces apoptosis in intracranial GBM xenografts. The combination of scL-p53 and TMZ increased the antitumor efficacy of TMZ with enhanced survival benefit in a mouse model of highly TMZ-resistant GBM. scL-p53 also sensitized both CSCs and bulk tumor cells to TMZ, increasing apoptosis. These results suggest that combining scL-p53 with standard TMZ treatment could be a more effective therapy for GBM.
Figures







Similar articles
-
Encapsulation of temozolomide in a tumor-targeting nanocomplex enhances anti-cancer efficacy and reduces toxicity in a mouse model of glioblastoma.Cancer Lett. 2015 Dec 1;369(1):250-8. doi: 10.1016/j.canlet.2015.08.022. Epub 2015 Sep 2. Cancer Lett. 2015. PMID: 26325605 Free PMC article.
-
A tumor-targeting p53 nanodelivery system limits chemoresistance to temozolomide prolonging survival in a mouse model of glioblastoma multiforme.Nanomedicine. 2015 Feb;11(2):301-11. doi: 10.1016/j.nano.2014.09.005. Epub 2014 Sep 18. Nanomedicine. 2015. PMID: 25240597 Free PMC article.
-
Effect of lomeguatrib-temozolomide combination on MGMT promoter methylation and expression in primary glioblastoma tumor cells.Tumour Biol. 2013 Jun;34(3):1935-47. doi: 10.1007/s13277-013-0738-7. Epub 2013 Mar 22. Tumour Biol. 2013. PMID: 23519841
-
Chemoresistance of glioblastoma cancer stem cells--much more complex than expected.Mol Cancer. 2011 Oct 11;10:128. doi: 10.1186/1476-4598-10-128. Mol Cancer. 2011. PMID: 21988793 Free PMC article. Review.
-
Potential Strategies Overcoming the Temozolomide Resistance for Glioblastoma.Neurol Med Chir (Tokyo). 2018 Oct 15;58(10):405-421. doi: 10.2176/nmc.ra.2018-0141. Epub 2018 Sep 21. Neurol Med Chir (Tokyo). 2018. PMID: 30249919 Free PMC article. Review.
Cited by
-
A receptor-mediated landscape of druggable and targeted nanomaterials for gliomas.Mater Today Bio. 2023 May 19;20:100671. doi: 10.1016/j.mtbio.2023.100671. eCollection 2023 Jun. Mater Today Bio. 2023. PMID: 37273792 Free PMC article. Review.
-
A stapled peptide antagonist of MDM2 carried by polymeric micelles sensitizes glioblastoma to temozolomide treatment through p53 activation.J Control Release. 2015 Nov 28;218:29-35. doi: 10.1016/j.jconrel.2015.09.061. Epub 2015 Sep 30. J Control Release. 2015. PMID: 26428461 Free PMC article.
-
Targeting transferrin receptor delivery of temozolomide for a potential glioma stem cell-mediated therapy.Oncotarget. 2017 Aug 10;8(43):74451-74465. doi: 10.18632/oncotarget.20165. eCollection 2017 Sep 26. Oncotarget. 2017. PMID: 29088799 Free PMC article.
-
A Novel P53 Nanomedicine Reduces Immunosuppression and Augments Anti-PD-1 Therapy for Non-Small Cell Lung Cancer in Syngeneic Mouse Models.Cells. 2022 Oct 31;11(21):3434. doi: 10.3390/cells11213434. Cells. 2022. PMID: 36359830 Free PMC article.
-
Tumor-targeted nanotherapeutics: overcoming treatment barriers for glioblastoma.Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017 Jul;9(4):10.1002/wnan.1439. doi: 10.1002/wnan.1439. Epub 2016 Nov 4. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2017. PMID: 27813323 Free PMC article. Review.
References
-
- Nagasawa D. T.; Chow F.; Yew A.; Kim W.; Cremer N.; Yang I. Temozolomide and Other Potential Agents for the Treatment of Glioblastoma Multiforme. Neurosurg. Clin. North Am. 2012, 23, 307–322. - PubMed
-
- Vredenburgh J. J.; Desjardins A.; Herndon J. E. 2nd; Dowell J. M.; Reardon D. A.; Quinn J. A.; Rich J. N.; Sathornsumetee S.; Gururangan S.; Wagner M.; et al. Phase II Trial of Bevacizumab and Irinotecan in Recurrent Malignant Glioma. Clin. Cancer Res. 2007, 13, 1253–1259. - PubMed
-
- Hou L. C.; Veeravagu A.; Hsu A. R.; Tse V. C. Recurrent Glioblastoma Multiforme: a Review of Natural History and Management Options. Neurosurg. Focus 2006, 20, E5. - PubMed
-
- Johannessen T. C.; Bjerkvig R.; Tysnes B. B. DNA Repair and Cancer Stem-Like Cells—Potential Partners in Glioma Drug Resistance?. Cancer Treat. Rev. 2008, 34, 558–567. - PubMed
-
- Chamberlain M. C. Temozolomide: Therapeutic Limitations in the Treatment of Adult High-Grade Gliomas. Expert Rev. Neurother. 2010, 10, 1537–1544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

