Role of Fc in antibody-mediated protection from ricin toxin
- PMID: 24811206
- PMCID: PMC4052250
- DOI: 10.3390/toxins6051512
Role of Fc in antibody-mediated protection from ricin toxin
Abstract
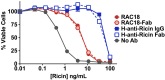
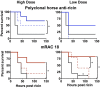
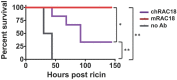
We have studied the role of the antibody (Ab) Fc region in mediating protection from ricin toxicity. We compared the in vitro and in vivo effects of intact Ig and of Fab fragments derived from two different neutralizing Ab preparations, one monoclonal, the other polyclonal. Consistent results were obtained from each, showing little difference between Ig and Fab in terms of antigen binding and in vitro neutralization, but with relatively large differences in protection of animals. We also studied whether importing Ab into the cell by Fc receptors enhanced the intracellular neutralization of ricin toxin. We found that the imported Ab was found in the ER and Golgi, a compartment traversed by ricin, as it traffics through the cell, but intracellular Ab did not contribute to the neutralization of ricin. These results indicate that the Fc region of antibody is important for in vivo protection, although the mechanism of enhanced protection by intact Ig does not appear to operate at the single cell level. When using xenogeneic antibodies, the diminished immunogenicity of Fab/F(ab')2 preparations should be balanced against possible loss of protective efficacy.
Figures




Similar articles
-
Antibody-mediated inhibition of ricin toxin retrograde transport.mBio. 2014 Apr 8;5(2):e00995. doi: 10.1128/mBio.00995-13. mBio. 2014. PMID: 24713323 Free PMC article.
-
Contribution of Fc fragment of monoclonal antibodies to tetanus toxin neutralization.Neurotox Res. 2020 Mar;37(3):578-586. doi: 10.1007/s12640-019-00124-9. Epub 2019 Nov 13. Neurotox Res. 2020. PMID: 31721050
-
Antibody to ricin a chain hinders intracellular routing of toxin and protects cells even after toxin has been internalized.PLoS One. 2013 Apr 24;8(4):e62417. doi: 10.1371/journal.pone.0062417. Print 2013. PLoS One. 2013. PMID: 23638075 Free PMC article.
-
Update on Fc-Mediated Antibody Functions Against HIV-1 Beyond Neutralization.Front Immunol. 2019 Dec 18;10:2968. doi: 10.3389/fimmu.2019.02968. eCollection 2019. Front Immunol. 2019. PMID: 31921207 Free PMC article. Review.
-
Extra-Neutralizing FcR-Mediated Antibody Functions for a Universal Influenza Vaccine.Front Immunol. 2019 Mar 18;10:440. doi: 10.3389/fimmu.2019.00440. eCollection 2019. Front Immunol. 2019. PMID: 30949165 Free PMC article. Review.
Cited by
-
Generation of Highly Efficient Equine-Derived Antibodies for Post-Exposure Treatment of Ricin Intoxications by Vaccination with Monomerized Ricin.Toxins (Basel). 2018 Nov 12;10(11):466. doi: 10.3390/toxins10110466. Toxins (Basel). 2018. PMID: 30424519 Free PMC article.
-
Parenteral Exposure of Mice to Ricin Toxin Induces Fatal Hypoglycemia by Cytokine-Mediated Suppression of Hepatic Glucose-6-Phosphatase Expression.Toxins (Basel). 2022 Nov 23;14(12):820. doi: 10.3390/toxins14120820. Toxins (Basel). 2022. PMID: 36548717 Free PMC article.
-
Post-Exposure Anti-Ricin Treatment Protects Swine Against Lethal Systemic and Pulmonary Exposures.Toxins (Basel). 2020 May 28;12(6):354. doi: 10.3390/toxins12060354. Toxins (Basel). 2020. PMID: 32481526 Free PMC article.
-
Intracellular Transport and Cytotoxicity of the Protein Toxin Ricin.Toxins (Basel). 2019 Jun 18;11(6):350. doi: 10.3390/toxins11060350. Toxins (Basel). 2019. PMID: 31216687 Free PMC article. Review.
-
Stability of isolated antibody-antigen complexes as a predictive tool for selecting toxin neutralizing antibodies.MAbs. 2017 Jan;9(1):43-57. doi: 10.1080/19420862.2016.1236882. Epub 2016 Sep 23. MAbs. 2017. PMID: 27660893 Free PMC article.
References
-
- Parham P. The Immune System. 3rd ed. Garland Publishing; New York, NY, USA: 2009.
-
- Janeway C.A., Travers P., Walport M., Shlomchik M. The Immune System in Health and Disease. 7th ed. Garland Publishing; New York, NY, USA: 2008. Immunobiology.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

