Sorting nexin 27 regulation of G protein-gated inwardly rectifying K⁺ channels attenuates in vivo cocaine response
- PMID: 24811384
- PMCID: PMC4141045
- DOI: 10.1016/j.neuron.2014.03.011
Sorting nexin 27 regulation of G protein-gated inwardly rectifying K⁺ channels attenuates in vivo cocaine response
Abstract
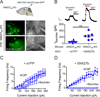
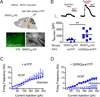
The subcellular pathways that regulate G protein-gated inwardly rectifying potassium (GIRK or Kir3) channels are important for controlling the excitability of neurons. Sorting nexin 27 (SNX27) is a PDZ-containing protein known to bind GIRK2c/GIRK3 channels, but its function in vivo is poorly understood. Here, we investigated the role of SNX27 in regulating GIRK currents in dopamine (DA) neurons of the ventral tegmental area (VTA). Mice lacking SNX27 in DA neurons exhibited reduced GABABR-activated GIRK currents but had normal Ih currents and DA D2R-activated GIRK currents. Expression of GIRK2a, an SNX27-insensitive splice variant, restored GABABR-activated GIRK currents in SNX27-deficient DA neurons. Remarkably, mice with significantly reduced GABABR-activated GIRK currents in only DA neurons were hypersensitive to cocaine and could be restored to a normal locomotor response with GIRK2a expression. These results identify a pathway for regulating excitability of VTA DA neurons, highlighting SNX27 as a promising target for treating addiction.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
A Role for the GIRK3 Subunit in Methamphetamine-Induced Attenuation of GABAB Receptor-Activated GIRK Currents in VTA Dopamine Neurons.J Neurosci. 2016 Mar 16;36(11):3106-14. doi: 10.1523/JNEUROSCI.1327-15.2016. J Neurosci. 2016. PMID: 26985023 Free PMC article.
-
GIRK currents in VTA dopamine neurons control the sensitivity of mice to cocaine-induced locomotor sensitization.Proc Natl Acad Sci U S A. 2018 Oct 2;115(40):E9479-E9488. doi: 10.1073/pnas.1807788115. Epub 2018 Sep 18. Proc Natl Acad Sci U S A. 2018. PMID: 30228121 Free PMC article.
-
GIRK Channel Activity in Dopamine Neurons of the Ventral Tegmental Area Bidirectionally Regulates Behavioral Sensitivity to Cocaine.J Neurosci. 2019 May 8;39(19):3600-3610. doi: 10.1523/JNEUROSCI.3101-18.2019. Epub 2019 Mar 5. J Neurosci. 2019. PMID: 30837265 Free PMC article.
-
G Protein-Gated Potassium Channels: A Link to Drug Addiction.Trends Pharmacol Sci. 2017 Apr;38(4):378-392. doi: 10.1016/j.tips.2017.01.007. Epub 2017 Feb 7. Trends Pharmacol Sci. 2017. PMID: 28188005 Free PMC article. Review.
-
G protein-gated inwardly rectifying K+ (GIRK/Kir3) channels: Molecular, cellular, and subcellular diversity.Histol Histopathol. 2025 May;40(5):597-620. doi: 10.14670/HH-18-822. Epub 2024 Sep 26. Histol Histopathol. 2025. PMID: 39434650 Review.
Cited by
-
A Role for the GIRK3 Subunit in Methamphetamine-Induced Attenuation of GABAB Receptor-Activated GIRK Currents in VTA Dopamine Neurons.J Neurosci. 2016 Mar 16;36(11):3106-14. doi: 10.1523/JNEUROSCI.1327-15.2016. J Neurosci. 2016. PMID: 26985023 Free PMC article.
-
GPCR Signaling and Trafficking: The Long and Short of It.Trends Endocrinol Metab. 2017 Mar;28(3):213-226. doi: 10.1016/j.tem.2016.10.007. Epub 2016 Nov 23. Trends Endocrinol Metab. 2017. PMID: 27889227 Free PMC article. Review.
-
Selective targeting of M-type potassium Kv 7.4 channels demonstrates their key role in the regulation of dopaminergic neuronal excitability and depression-like behaviour.Br J Pharmacol. 2017 Dec;174(23):4277-4294. doi: 10.1111/bph.14026. Epub 2017 Oct 19. Br J Pharmacol. 2017. PMID: 28885682 Free PMC article.
-
Sorting Nexin 27 as a potential target in G protein‑coupled receptor recycling for cancer therapy (Review).Oncol Rep. 2020 Nov;44(5):1779-1786. doi: 10.3892/or.2020.7766. Epub 2020 Sep 14. Oncol Rep. 2020. PMID: 33000258 Free PMC article. Review.
-
Behavioral and Genetic Evidence for GIRK Channels in the CNS: Role in Physiology, Pathophysiology, and Drug Addiction.Int Rev Neurobiol. 2015;123:279-313. doi: 10.1016/bs.irn.2015.05.016. Epub 2015 Jun 22. Int Rev Neurobiol. 2015. PMID: 26422988 Free PMC article. Review.
References
-
- Arora D, Hearing M, Haluk DM, Mirkovic K, Fajardo-Serrano A, Wessendorf MW, Watanabe M, Lujan R, Wickman K. Acute cocaine exposure weakens GABA(B) receptor-dependent G-protein-gated inwardly rectifying K+ signaling in dopamine neurons of the ventral tegmental area. J Neurosci. 2011;31:12251–12257. - PMC - PubMed
-
- Balana B, Maslennikov I, Kwiatkowski W, Stern KM, Bahima L, Choe S, Slesinger PA. Mechanism underlying selective regulation of G protein-gated inwardly rectifying potassium channels by the psychostimulant-sensitive sorting nexin 27. Proceedings of the National Academy of Sciences. 2011;108:5831–5836. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

