Identification of Hic-5 as a novel scaffold for the MKK4/p54 JNK pathway in the development of abdominal aortic aneurysms
- PMID: 24811612
- PMCID: PMC4309060
- DOI: 10.1161/JAHA.113.000747
Identification of Hic-5 as a novel scaffold for the MKK4/p54 JNK pathway in the development of abdominal aortic aneurysms
Abstract
Background: Although increased amounts of reactive oxygen species in the pathogenesis of abdominal aortic aneurysm (AAA) are well documented, the precise molecular mechanisms by which reactive oxygen species induce AAAs have not been fully elucidated. This study focused on the role of hydrogen peroxide-inducible clone 5 (Hic-5), which is induced by hydrogen peroxide and transforming growth factor-β, in the cellular signaling of AAA pathogenesis.
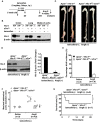
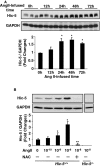
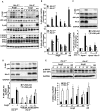
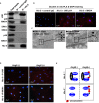
Methods and results: Using the angiotensin II-induced AAA model in Apoe(-/-) mice, we showed that Apoe(-/-)Hic-5(-/-) mice were completely protected from AAA formation and aortic rupture, whereas Apoe(-/-) mice were not. These features were similarly observed in smooth muscle cell-specific Hic-5-deficient mice. Furthermore, angiotensin II treatment induced Hic-5 expression in a reactive oxygen species-dependent manner in aortic smooth muscle cells in the early stage of AAA development. Mechanistic studies revealed that Hic-5 interacted specifically with c-Jun N-terminal kinase p54 and its upstream regulatory molecule mitogen-activated protein kinase kinase 4 as a novel scaffold protein, resulting in the expression of membrane type 1 matrix metalloproteinase and matrix metalloproteinase 2 activation in aortic smooth muscle cells.
Conclusion: Hic-5 serves as a novel scaffold protein that specifically activates the mitogen-activated protein kinase kinase 4/p54 c-Jun N-terminal kinase pathway, thereby leading to the induction and activation of matrix metalloproteinases in smooth muscle cells and subsequent AAA formation. Our study provided a novel therapeutic option aimed at inhibiting the mitogen-activated protein kinase kinase 4-Hic-5-p54 c-Jun N-terminal kinase pathway in the vessel wall, particularly through Hic-5 inhibition, which may be used to produce more precise and effective therapies.
Keywords: Hic‐5; JNK‐signaling scaffold protein; aneurysm; smooth muscle.
Figures



Similar articles
-
Fucoidan attenuates angiotensin II-induced abdominal aortic aneurysms through the inhibition of c-Jun N-terminal kinase and nuclear factor κB activation.J Vasc Surg. 2018 Dec;68(6S):72S-81S.e1. doi: 10.1016/j.jvs.2017.09.042. Epub 2017 Dec 28. J Vasc Surg. 2018. PMID: 29290496
-
Cortistatin attenuates angiotensin II-induced abdominal aortic aneurysm through inactivation of the ERK1/2 signaling pathways.Biochem Biophys Res Commun. 2018 Jan 8;495(2):1801-1806. doi: 10.1016/j.bbrc.2017.12.033. Epub 2017 Dec 7. Biochem Biophys Res Commun. 2018. PMID: 29225168
-
Ginsenoside Rb1 attenuates angiotensin II-induced abdominal aortic aneurysm through inactivation of the JNK and p38 signaling pathways.Vascul Pharmacol. 2015 Oct;73:86-95. doi: 10.1016/j.vph.2015.04.003. Epub 2015 Apr 22. Vascul Pharmacol. 2015. PMID: 25912763
-
Transforming growth factor-β and abdominal aortic aneurysms.Cardiovasc Pathol. 2013 Mar-Apr;22(2):126-32. doi: 10.1016/j.carpath.2012.07.005. Epub 2012 Sep 6. Cardiovasc Pathol. 2013. PMID: 22959236 Review.
-
[Effects of Leptin and Leptin-associated Signaling Pathways on the Occurrence and Progression of Abdominal Aortic Aneurysms].Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2022 Dec;44(6):1075-1081. doi: 10.3881/j.issn.1000-503X.14303. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2022. PMID: 36373641 Review. Chinese.
Cited by
-
Angiotensin II Signal Transduction: An Update on Mechanisms of Physiology and Pathophysiology.Physiol Rev. 2018 Jul 1;98(3):1627-1738. doi: 10.1152/physrev.00038.2017. Physiol Rev. 2018. PMID: 29873596 Free PMC article. Review.
-
Physiological and pathological roles of Hic‑5 in several organs (Review).Int J Mol Med. 2022 Nov;50(5):138. doi: 10.3892/ijmm.2022.5194. Epub 2022 Oct 12. Int J Mol Med. 2022. PMID: 36222304 Free PMC article. Review.
-
Normal Platelet Integrin Function in Mice Lacking Hydrogen Peroxide-Induced Clone-5 (Hic-5).PLoS One. 2015 Jul 14;10(7):e0133429. doi: 10.1371/journal.pone.0133429. eCollection 2015. PLoS One. 2015. PMID: 26172113 Free PMC article.
-
Knockdown of mechanosensitive adaptor Hic-5 ameliorates post-traumatic osteoarthritis in rats through repression of MMP-13.Sci Rep. 2023 May 8;13(1):7446. doi: 10.1038/s41598-023-34659-x. Sci Rep. 2023. PMID: 37156857 Free PMC article.
-
The impact of stromal Hic-5 on the tumorigenesis of colorectal cancer through lysyl oxidase induction and stromal remodeling.Oncogene. 2018 Mar;37(9):1205-1219. doi: 10.1038/s41388-017-0033-y. Epub 2017 Dec 15. Oncogene. 2018. PMID: 29242607
References
-
- Stanley JC, Barnes RW, Ernst CB, Hertzer NR, Mannick JA, Moore WS. Vascular surgery in the united states: workforce issues. Report of the society for vascular surgery and the international society for cardiovascular surgery, north american chapter, committee on workforce issues. J Vasc Surg. 1996; 23:172-181. - PubMed
-
- McCormick ML, Gavrila D, Weintraub NL. Role of oxidative stress in the pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2007; 27:461-469. - PubMed
-
- Miller FJ, Jr, Sharp WJ, Fang X, Oberley LW, Oberley TD, Weintraub NL. Oxidative stress in human abdominal aortic aneurysms: a potential mediator of aneurysmal remodeling. Arterioscler Thromb Vasc Biol. 2002; 22:560-565. - PubMed
-
- Gavazzi G, Deffert C, Trocme C, Schappi M, Herrmann FR, Krause KH. NOX1 deficiency protects from aortic dissection in response to angiotensin II. Hypertension. 2007; 50:189-196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

