A high-content morphological screen identifies novel microRNAs that regulate neuroblastoma cell differentiation
- PMID: 24811707
- PMCID: PMC4058022
- DOI: 10.18632/oncotarget.1703
A high-content morphological screen identifies novel microRNAs that regulate neuroblastoma cell differentiation
Abstract
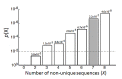
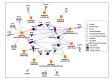
Neuroblastoma, the most common extracranial solid tumor of childhood, arises from neural crest cell precursors that fail to differentiate. Inducing cell differentiation is an important therapeutic strategy for neuroblastoma. We developed a direct functional high-content screen to identify differentiation-inducing microRNAs, in order to develop microRNA-based differentiation therapy for neuroblastoma. We discovered novel microRNAs, and more strikingly, three microRNA seed families that induce neuroblastoma cell differentiation. In addition, we showed that microRNA seed families were overrepresented in the identified group of fourteen differentiation-inducing microRNAs, suggesting that microRNA seed families are functionally more important in neuroblastoma differentiation than microRNAs with unique sequences. We further investigated the differentiation-inducing function of the microRNA-506-3p/microRNA-124-3p seed family, which was the most potent inducer of differentiation. We showed that the differentiation-inducing function of microRNA-506-3p/microRNA-124-3p is mediated, at least partially, by down-regulating expression of their targets CDK4 and STAT3. We further showed that expression of miR-506-3p, but not miR-124-3p, is dramatically upregulated in differentiated neuroblastoma cells, suggesting the important role of endogenous miR-506-3p in differentiation and tumorigenesis. Overall, our functional screen on microRNAs provided the first comprehensive analysis on the involvements of microRNA species in neuroblastoma cell differentiation and identified novel differentiation-inducing microRNAs. Further investigations are certainly warranted to fully characterize the function of the identified microRNAs in order to eventually benefit neuroblastoma therapy.
Figures

References
-
- Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–2120. - PubMed
-
- Park JR, Eggert A, Caron H. Neuroblastoma: biology, prognosis, and treatment. Hematol Oncol Clin North Am. 2010;24(1):65–86. - PubMed
-
- Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3(3):203–216. - PubMed
-
- Reynolds CP. Differentiating agents in pediatric malignancies: retinoids in neuroblastoma. Curr Oncol Rep. 2000;2(6):511–518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

